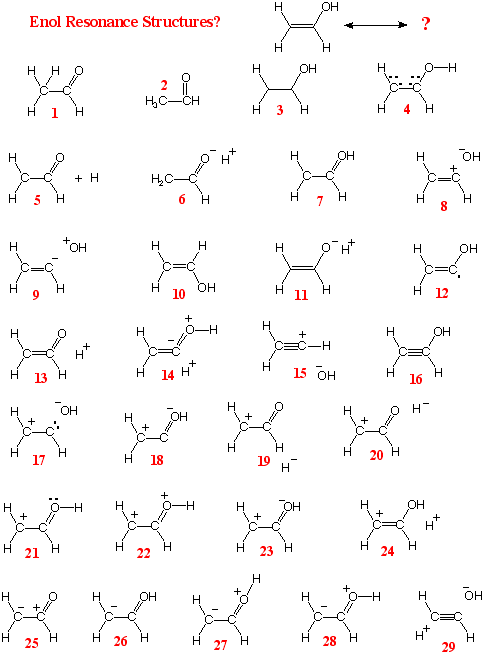
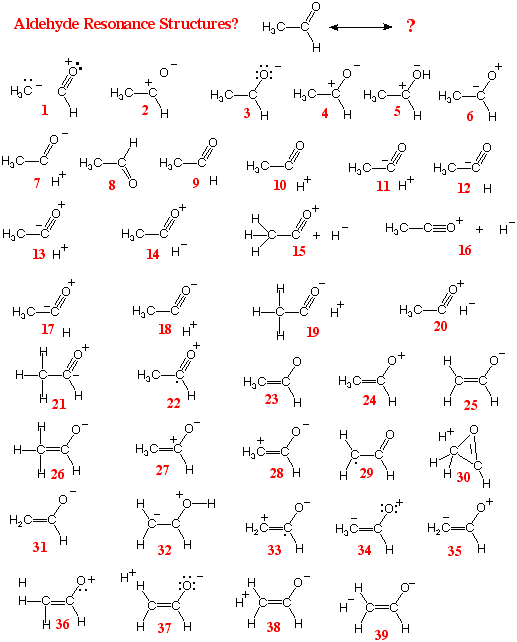Resonance Structures of
CH3CHO and
of
CH2=CHOH
The following are two parts of a question on the
Fall Term Final Exam for 2001-2002
|
Quest. 7 c)
Draw the best alternative resonance
structure you can imagine for A, and the best for B. In each
case specify which OMO and which UMO are being mixed to
generate the resonance structure.
|

|
|
Answer:
(A) The HOMO
is
sC-H
; the LUMO is p*C=O
(B) The HOMO is
nO ; the LUMO is p*C=C
|

|
|
Quest.
7
d)
Which molecule, A or B, should be more
stabilized by resonance? Explain your
thinking.
|
|
Answer:
B should be more
stabilized. It has both an unusually high OMO
(nO) and an unusually low UMO
(p*C=C).
A has an even lower UMO
(p*C=O),
but sC-H
is a pretty poor excuse for a OMO.
Still such resonance structures explain stabilization due to
"hyperconjugation".
|
Among 57 exam papers there were 39
different answers for part A (5 of them sensible) and
29 different answers for part B (6 of them sensible)!
This suggests that some review of the use of resonance structures is
in order before applying these ideas in the second semester. One way
to review would to evaluate all of the resonance structures that
were proposed, finding the correct ones, and identifying what is
wrong with those that are not sensible. I suggest that you do
this, individually or in groups, until you are satisfied that, faced
with the same type of problem in the future, you will have no
difficulties.
After initially being rocked back on my heels by
the variety of incorrect resonance structures that were proposed, I
decided I was not too disappointed for two reasons.
First because we did not really drill on
the use of resonance structures after the first week of class,
choosing to focus on an orbital view of structure and reactivity,
and the exam question was one which required a rapid response and
included an unfamiliar "hyperconjugated" structure.
Second, because in many cases correct use of
resonance structures depends on having mastered lore, and we
haven't dealt yet with the lore of aldehydes and enols. Having
learned facts allows you to pretend to yourself that you are
predicting things from first principles, when in fact you are just
restating or generalizing empirically from facts you already know.
There is nothing fundamentally wrong with this approach, and in
fact a version of resonance theory known as "mesomerism" was
developed by organic chemists before they knew anything of quantum
mechanics. You will get better at this kind of theory as time goes
on and you learn more facts.
You can get a long way without knowing all the
lore by using quantum mechanical ideas you have already mastered
- knowing what bonds are and how HOMOs mix with LUMOs. As you will
learn second semester, organic chemistry cannot be mastered by using
fundamental theory alone, lore is essential in dealing with complex
reality. But chemical lore is easire to organize and masterif you
have guidance from bonding theory.
Still, it is important, both for everyday
convenience and for communicating with others who put more reliance
on resonance structures, to be able to manipulate such structures
properly. Many individuals would be scandalized by some
of the incorrect structures that appeared on the exam papers.
One of the most common kinds of resonance
structure involves replacing a single covalent bond (or one member of
a double bond) between atoms of different electronegativity by
complementary charges on the bonded atoms. This indicates bond
polarity due to uneven sharing of the bonding electron pair (because
of poor energy match). However this kind of resonance doesn't lend
itself very well to answering the question above, which asks for
identification of HOMO/LUMO interactions that give rise to the
resonance structures. Substantial credit was given for such polar
resonance forms, but better answers were available.
The following criteria allow you to evaluate
proposed resonance structures:
1) Two resonance structures must
involve the same set of atoms (don't add or lose
atoms)
2) The structures must have the same
net charge (don't add or lose electrons)
3) The atoms must be in the same
position (the structures show different bonding schemes for a
single set of atomic positions)
4) The number of electrons associated
with an atom should not exceed the capacity of its valence
orbitals (octet for 2nd-row elements)
5) Be sure that the formal charge on
an atom reflects its nuclear charge, its half-interest in
bonding pairs, and its possession of unshared pairs or odd
electrons.
6) Preserve the maximum number of
bonds. Relocate bonds, but don't lose bonds without having a
very good reason for doing so (bond polarity maybe)
7) When polar forms are used to replace
bonds, be sure that the charge distribution properly reflects
relative electronegativity (energy mismatch).
8)
If the structure reflects
OMO/UMO mixing within the molecule, be sure the two orbitals
overlap (s
and
p
orbitals on the same atom are orthogonal).
Point 8 is highlighted in
the above list, because it is the one question 7 was aimed at. The
other criteria allow you to dismiss erroneous structures, but
effective mixing between localized OMOs and UMOs is the main
reason one must consider resonance structures altogether.
First we construct a
preliminary bonding scheme for a molecule based on
localized orbitals formed as bonding/antibonding pairs by
overlapping of two AOs on adjacent atoms. The AOs may be
hybridized, and the scheme may include unpaired AOs with one or
two electrons (unshared pairs or radicals) or none at all, as
necessary. The bonds may be polarized because of energy mismatch
between the two AOs of a bond (a primitive type of resonance
writes polar structures to denote this fact).
Now the idea is to identify
the high OMOs and low UMOs of this preliminary structure. If they
overlap, the preliminary structure is inadequate for predicting
the molecule's properties. The true molecule will have
different properties (lower energy especially) than predicted by
the preliminary structure because of the mixing between these
localized MOs. If there are no important OMO/UMO interactions, the
preliminary structure should be adequate.
The higher the energy of the
preliminary OMO, and the lower the energy of the preliminary UMO,
the more drastic the alteration in properties due to their
overlapping, and the more important the resonance structure. The
resonance structure will show increased bonding in the overlap
region between OMO and UMO. It will show decreased bonding where
there was overlap within the original OMO, or a node within the
original UMO. It will show increased bonding where there was a
node within the original OMO, or overlap within the original
UMO.
With practice applying criteria
1-7 becomes intuitively obvious. Applying criterion 8 typically
requires some thought.
Working conscientiously through the checklists
below should help build your skills and confidence for understanding
and using resonance structures. Among all the
resonance structures there is only one really great one, the one
indicated in B above. This was the point of question 7d.
copyright 2002 J. M. McBride