|
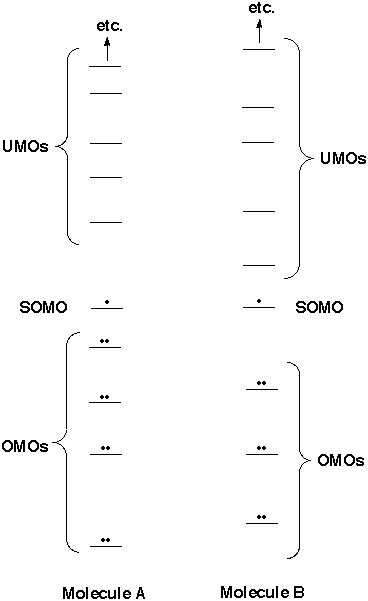
To get an idea of the tendency of the two species (let's call them molecules A and B) to stick together, we can check the interactions of all pairs of orbitals (their overlap and energy match) and see whether there is a way to lower the total energy from that of the separated molecules. Each molecule has a certain number of (doubly) occupied molecular orbitals (OMOs), an infinite number of unoccupied molecular orbitals (UMOs) and, if it has an odd number of electrons, a singly occupied molecular orbital (SOMO). Of course the OMOs are lower in energy than the SOMO, which is lower in energy than the UMOs. (That is why they are occupied or not.) Each molecule has so many orbitals that it looks like a daunting task to consider all possible pairs of orbitals between A and B and to decide what the overall effect on energy should be of bringing A and B together. Fortunately most interactions are irrelevant, and usually it is possible to focus on just one or two orbital interactions to decide whether two molecules should react with one another. Below we consider possible pairs and show why almost all of them can be disregarded. |
 |
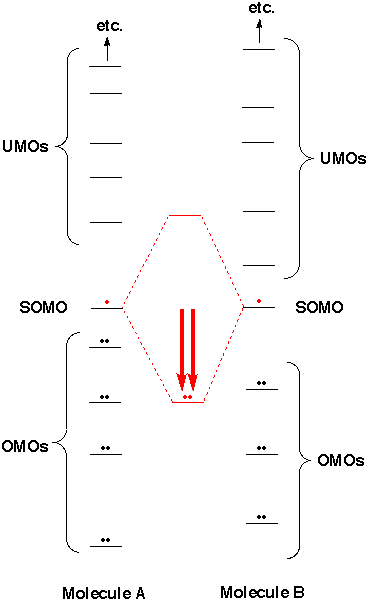 |
Usually there are no SOMOs, so we don't need to consider them. When two SOMOs come together and overlap, there is a dramatic stabilization of two electrons. The strong bond formed is typically worth about 100 kcal/mole (like the simplest of all cases - two H atoms forming an H2 molecule). Because this type of stabilization is so favorable, "free radicals" (that is, species with SOMOs) typically react very rapidly with one another. One almost never finds free radicals in appreciable concentration. They would already have found, and reacted with, one another. They are observed only in very low concentration (as in short-lived reactive intermediates), or trapped in solids where they can't move to find one another (as in toast), or at fabulously high temperatures (as in flames). If there are SOMOs that can overlap, we can ignore everything else. The molecules will react by forming a bond between two SOMOs. [Cases where SOMOs can't overlap well provide the exceptions that prove the rule. There is a class of rare molecules, that however includes O2, which have several SOMOs.] |
|
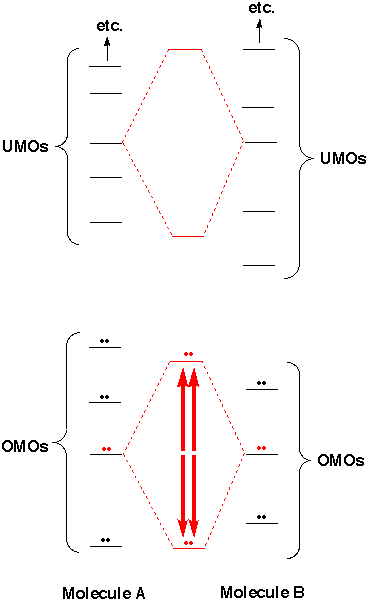
Two UMOs may overlap and give new orbitals of different energy, but if there are no electrons to go in the orbitals, we don't care what these possible energies for electrons are. For most purposes we can ignore UMO-UMO interaction. [Of course if a favorable combination of UMOs came out to be lower in energy than some OMO combination, the electrons would shift from the latter to the former, and we would then care about the UMO-UMO energy. However, the UMOs are usually so far above the OMOs that this almost never happens. Even after mixing with one another in a favorable way, they are still higher than the OMOs.] When two OMOs mix, two electrons go down in energy and two go up. To a first approximation these shifts cancel one another, so for most purposes we can ignore OMO-OMO interaction. If we want to be picky we can note that in fact the "antibonding" electrons go up in energy a little more than the bonding electrons go down in energy, so the overlap of filled orbitals is slightly unfavorable on balance and the molecules should repel one another. To form bonds there must be stabilizing interactions that are strong enough to overcome this source of repulsion. One such interaction is that of SOMOs with one another (above), but if there are enough OMO-OMO repulsions, the SOMO attraction might fail, and free radicals could be observed in substantial concentration. If we were interested in the energy of individual orbitals, rather than in total energy, we would be interested in OMO-OMO or UMO-UMO mixing. This is important for light absorption and ionization processes. However, the resulting energy shifts are usually much smaller than those from SOMO-SOMO interactions, because the overlap between orbitals that are not pointing toward one another is modest. Typically the overlap between different bonding OMOs is less than 10% as large as that between appropriately hybridized atomic orbitals. |
 |
 |
When an OMO overlaps with an UMO its electrons are definitely stabilized, which would be a source of bonding. However, as mentioned above, most UMOs are much higher in energy than most OMOs, so the energy match is usually terrible. This means the the best combination of overlapping OMO and UMO looks almost like the OMO both in shape and in energy. We can ignore most OMO-UMO interactions. They will not be stabilizing enough to overcome the OMO-OMO repulsions mentioned in the preceding frame. |
|
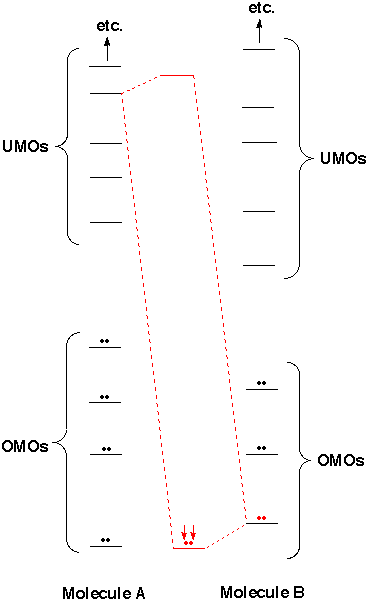
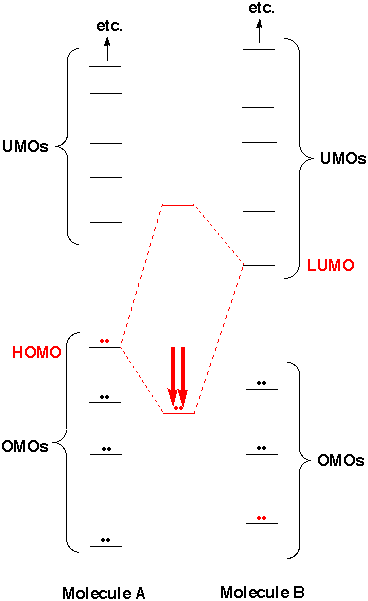
Every molecule has a highest occupied molecular orbital (HOMO) and a lowest unoccupied molecular orbital (LUMO). Sometimes the HOMO is not only the highest in the molecule but is UNUSUALLY high, like the one indicated in red on the near right. Sometimes the LUMO is UNUSUALLY low, like the one indicated in red on the far right. Because there is reasonable energy match between this HOMO and this LUMO, there can be substantial lowering in energy. If this is enough to overcome the repulsion from interaction of other OMOs with one another, a reaction occurs. What is necessary for reaction is an unusually high HOMO in one reaction partner and an unusually low LUMO in the other. Note that in the case on the right Molecule A is reactive because of its unusually high HOMO (its LUMO is nothing special). Molecule B is reactive because of its unusually low LUMO (its HOMO is nothing special). By predicting which molecules should have unusually high HOMOs and which should have unusually low LUMOs we can recognize functional groups, and predict which functional groups should react with one another. Once we learn to identify unusually high HOMOs and unusually low LUMOs we'll be in great shape.
|
 |
|
|
|
|
copyright 2001 J. M. McBride