What "a disordered arrangement"
is.
The answer to this semantic riddle involves
nomenclature.
It is true than any specific arrangement of
molecules can be considered every bit as "ordered" as any other
specific arrangement, but when Feynman speaks of "a disordered
arrangement", he is using the words as a code to mean the set of
all arrangements that do not correspond to some particular
ordered arrangement (or set of ordered arrangements) of
interest.
Obviously many more arrangements are lumped
together under the name "a disordered arrangement" than are included
under the name of any particular ordered arrangement. It is this
larger number of disordered arrangements that creates the entropic
bias.
Feynman goes on to write (p. I 46-7), "So we now have to
talk about what we mean by disorder and what we mean by order...
We measure 'disorder' by the number of ways that the insides
[of a grid containing black balls and white ones] can be
arranged, so that from the outside it looks the same."
[notice the work "looks". Eye of the beholder.]
Supplement: The Time
Problem
Whether this kind of entropic bias gives a
direction to the "arrow of time" is another
question.
The basis of irreversibility is randomness. If you
have some particular arrangement of particles and one of them
undergoes a random displacement, there is the possibility that the
same particle could undergo the reverse displacement and return the
system to the starting arrangement. After a random walk with an even
number of steps the original arrangement is always more likely than
any other specific arrangement. But the original arrangement becomes
increasingly less likely than the set of all other accessible
arrangements. Obviously, the more particles there are - the higher
the dimensionality of the problem - the less likely is return to the
original arrangement.
|
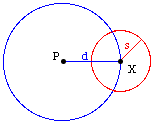
Consider two
dimensions.
A step of
length
s in an arbitrary direction
from position X not only ends up further from X, it is also
more likely to be away from any given point P than
toward it. (More of the red circle is outside the blue
circle than inside it.) The bias becomes stronger in higher
dimensions.
So when Nature is left to its own random
ways, the tendency is to move away from any specific
arrangement, whether one considers it "ordered" or
"disordered".
|
|
Unless you know the initial arrangement, there is no way to know
whether you are going forward in time and drifting away from it, or
going backward in time and drifting toward it.
Of course there are very few arrangements whose
overall order we can perceive (when the ancients found bears, a
scorpion, and a hunter among the stars, they were being quite
selective in which stars they considered). So if there is obvious
overall order, we can guess that it did not arise at random. There is
a clear time sequence between Humpty Dumpty and his
fragments.
Still, the tendency to move randomly away from an
ordered arrangement can be overwhelmed by a sufficiently strong
energetic bias for moving toward it - consider crystal formation or
the condensation of steam.
If the energetic bias is not strong enough, the
tendency of random steps to move away from any particular
arrangement, can make systems move uphill in energy. When water
evaporates at room temperature, or ice melts at 0░, the water
molecules have to pull energy from the surroundings in order to
overcome the mutual attraction they enjoy in the more ordered state.
(This is also what makes a rubber
band contract and what allows an adiabatic
demagnetization refrigerator to cool samples to 0.05 K, or even
much lower.)
In many cases of chemical interest, randomization
of the distribution of energy within a system is more important for
its time development than is the randomization of the spatial
distribution of particles, making the increase of any na´vely
perceived spatial "disorder" an unreliable measure of increasing
time. Supersaturated solutions do spontaneously
crystallize.
copyright 2002
J.M.McBride
