The "Liebig"
Condenser
(note the red lessons and
questions)
In discussing early 19th Century contributions that still provide
valuable lessons for chemistry, we have concentrated mostly on
elemental analysis (for example, Combustion
and Liebig's "Kaliapparat")
and the theories that sprang from it. Techniques for separation and
purification have always been equally important. Perhaps a brief
discussion of early tools for distillation will help make you think
about what we are doing, particular in the laboratory.
In the experimental techniques section of his "Traité
Élémentaire de Chimie"
(1789) Lavoisier
provided a discussion of purification by distillation
illustrated with etchings by Mme.
Lavoisier.
Here is some of what Lavoisier says about
"Simple Distillation":
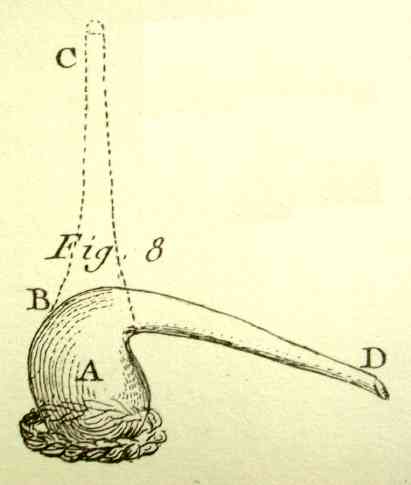
The simplest of all apparatus for distillation is a bottle
A, plate III, fig.8
[right], of which one bends
the neck of the glass itself from BC to BD. This bottle or
phial is called a "cornue";* one places it either in a
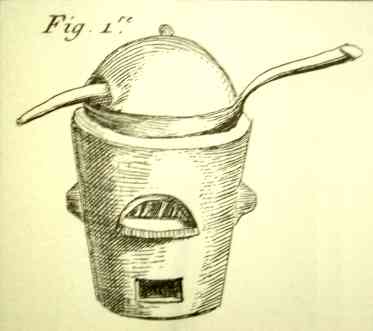
furnace as shown in plate XIII, fig 2, or in a sand
bath under a pottery lid, as shown in plate III, fig.
1 [below right]. To
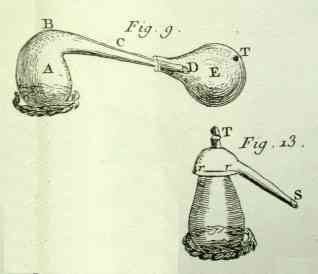
collect and condense the product, one fits the cornue with a
receiver E, plate III, fig. 9
[below], which one cements to it: sometimes,
especially in pharmaceutical operations one uses...a glass
alembic,** of which the top is a single piece, figure
13 [below].
But, since almost all distillations involve expansion of
vapors that could shatter the vessel, one must equip the
flask or the receiver E, fig. 9, with a little
hole T, to allow the escape of vapors. Thus one sees
that in this mode of distilling one loses any product
that is permanently gaseous, and even those
that, since they do not easily leave this state,
do not have time to be condensed inside the flask.
*) From French for "horn", cf.
cornucopia. More commonly the vessel is called a "retort"
from the Latin word meaning "twisted".
**) "Alembic" is from the Arabic "al
anbiq, the still" from the Greek
ambix,
ambix, head of a still. This is really ancient
technology.
Note the warning
to beginning organic students down through the centuries,
who have often overlooked the necessity of leaving the
little hole that keeps the still from "shattering", or at
least flying apart.
|
|
|
|
In the passage above, Lavoisier emphasizes the problem of losing
liquids that are difficult to condense during distillation with a
cornue/retort/alembic. He described some more elaborate equipment,
but there is a better solution.
The solution to this problem is to use a water-cooled condenser,
since water has a much greater capacity for removing heat than the
air that was used to cool the ancient vessels. The familiar
water-cooled condenser is traditionally referred to as a "Liebig
condenser".
In lab you use a glass-jacketed Liebig condenser with rubber
tubing to supply and drain water.
Liebig's own tin-jacketed condenser in his Giessen laboratory
[below] used an elevated bucket with a
tap to supply water to a funnel, which led it to the bottom of the
jacket. The water rose in the jacket to leave at the top and drip
into a second bucket, which could then be emptied into the top bucket
again for recycling.
|
You should wonder in looking at Liebig's setup, or while
connecting the tubing to your own condenser in lab,
"Why should the cooling water enter
at the bottom and leave at the top?" After all,
this was inconvenient and required Liebig to use an
extraordinarily long funnel.
Of course the lab manual tells you to do it this
way, but why? (click
here for answer)
In 1896 it was pointed out that Christian E. Weigel's
1770 M.D. thesis in Göttingen included a detailed
drawing (below right) of his
tin-jacketed "Liebig" condenser, which he used for
"Destillatio spiritus vini." This was 32 years before
Liebig's birth! Weigel also made a water-cooled condenser
from glass rather than tin.
Here is another example of how being in an
influential position, as Liebig was in his Giessen
teaching laboratory, or Kekulé in Ghent and Bonn,
can make someone "famous for being famous." People are no
more likely to refer to a Weigel condenser than they are
to refer to Couper (rather than Kekulé) structures
of organic molecules.
There had been one fairly minor improvement in the 60
years between Weigel's condenser, where water pours out of
the jacket (and perhaps down its bottom
side), and Liebig's, where it emerges at the top
through a cork into a curved tube that dumps it into the
bucket.
Think about how much of the inner tube is
covered in water, and about how you should turn the
condenser in your own distillations. Granted it doesn't
make much difference, but in principle
should you rotate your condenser
so that the rubber tubing leaves from the top or the
bottom side of the top end of the condenser?
[Think about where air
is in the jacket.]
|

|
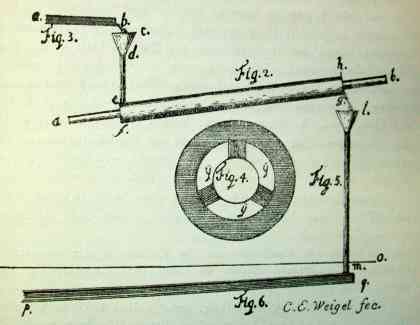
Weigel's Condenser. Fig 4 is a cross section of
the condenser showing the interior tube, the space for
water, and three struts supporting the outer
jacket.
|
copyright 2001
J.M.McBride