The "Kaliapparat", Liebig's Five-Bulb
Apparatus
The most important quantitative tools of 19th
century organic chemistry were the analytical balance and the
Five-Bulb (Fünfkugel) Apparatus, usually called the
"Kaliapparat" (interesting
etymology), which Justus Liebig invented in 1831.
These tools, together with well-chosen chemical transformations and
clever reasoning, allowed 19th Century chemists to determine the
nature of organic molecules and their reactions long before x-ray
diffraction, structural spectroscopy, and chemical quantum mechanics
were dreamt of.
|
By allowing efficient absorption of the
CO2 generated by combustion, the Kaliapparat made
it relatively easy to determine the amount of carbon
in a sample by weight.
Liebig had an analytical balance
(right), made by a local cabinet
maker named Hoss, with which he could weigh 100 grams to an
accuracy of 0.3 mg. If burning a half-gram sample produced
something like a gram of CO2, he could weigh it
to an accuracy of better than 0.1%.
The long arm of the balance swung slowly,
and the process of weighing was time consuming. Liebig could
smoke a cigar during the time it took to make a
high-precision weighing.
Problem:
Do you think you could measure the volume of
gaseous CO2 to 0.1% accuracy with
Prout's
apparatus? [Consider the
influence of temperature and atmospheric
pressure.]
|

|

|
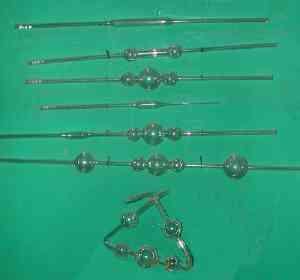
The delicate piece of glassware to the
left is more that 150 years old, the last example of a
Kaliapparat to survive from Liebig's laboratory. It is
displayed in the Liebig
Museum in Giessen, which is
reasonably called the "Birthplace of Modern Organic
Chemistry", because it is where Liebig taught from 1824
until 1852.
The Museum also has an exhibit, shown at
the right, illustrating the stages a glass blower would go
through in fabricating a Kaliapparat according to Liebig's
1837 instructions.
Students from all over the world flocked
to Liebig's Giessen lab to learn how to be organic chemists.
Of the first 61 Nobel Prizes in Chemistry, 44 were awarded
to scientific descendants of students from this
laboratory.
|
|

|
Not surprisingly many portraits of
Liebig, like the one on the right,
include a Kaliapparat (which in this 1852 photograph is connected
backwards, with the combustion gases entering the smaller bulb).
If you didn't click on the
Liebig
Museum link above, click now to
see an illustration showing 13 individuals in Liebig's lab
in 1840.
The 22-year-old dandy at the far
right is August Wilhelm Hofmann,
whom we'll meet later in the course.
The casual individual at the far left
(see detail left),
the only one who gets to hold a Kaliapparat in the illustration, is
Vicente Ortigosa, the first Mexican Ph.D. in organic chemistry, whose
analyses were so good that he is reported
to have corrected Liebig's errors. Here he is holding the Kaliapparat
upside down, although in a black and white sketch of the
same scene (below) he holds it right side up.
[Note the apparatus at the top of the Museum web
page.]
|

Click
here to see other portraits
of Liebig from the Museum.
The romantic 18-year-old is notable.
|
 |
Liebig's Kaliapparat remained in use for three
quarters of a century and became a badge of honor for organic
chemistry. Below it appears in the logo of the American Chemical
Society, on the southwest corner of Sterling Chemistry Laboratory,
and as a woodcarving in Yale's Chemistry Library. SCL was constructed
in 1923, when some chemists were still familiar with the Kaliapparat,
although it began disappearing from general use near the beginning of
the 20th Century with the development of microanalytical methods
involving samples 100 times smaller than Liebig's, balances 300 times
more sensitive, and absorption of CO2 on a solid rather
than in a solution. |

Logo of the American
Chemical Society
Incorporation of the Kaliapparat in the
logo was suggested by J. L. Smith, one of the founders of
the ACS who had been a Liebig student in 1842.
|

|

|
Etymology
of "Kali" and the Atomic Symbols K and Na
The Kaliapparat was so called in German because
the apparatus contained a solution of kali (or caustic potash,
i.e. KOH) to absorb CO2. The word kali derives
in the alchemical tradition from the Arabic qalay, "to fry or
roast in a pan", and al-qalay , "the substance that had been
roasted", that is alkali; al being the definite article
in Arabic (as in alchemy, alcohol, algebra, Alameda,
etc.).
The sense of alkali is that certain plants
were burned and their ashes were extracted with water, which was then
evaporated in large iron pots to leave al-kali or pot-ash. This
"fixed alkali" was mostly the carbonates of what we call alkali
metals. Heated further the fixed alkali could lose carbonate by
exchanging anions with lime (calcium hydroxide) to give "caustic
potash", which subsequently could lose water to give the metallic
oxides.
In the early 18th century it was realized that
there were different alkalies: mineral alkali (soda), vegetable
alkali (potash), and animal alkali (ammonia). The English word
soda derived from suwwad, the Arabic name of a plant of
which the ashes are rich in sodium carbonate. The name natron
for sodium carbonate came from the Arabic narn, Hebrew
neter, which derive from the Greek nitre (also the
source of the word nitrate).
During a single week in 1807 Humphrey Davy in
London, who had a humongous battery with 250 cells, electrolyzed both
potash and soda as solids to prepare two new elemental metals and
gave them the latinized names potassium and sodium. In
1813 Berzelius published in a British journal, Thomas Thomson's
Annals of Philosophy, his system
of atomic symbols as one- or
two-letter abbreviations of Latin names for the elements. In this
first paper he followed the British discoverer Davy in nomenclature
and abbreviated potassium and sodium as Po and So. But
within a year Berzelius decided in favor of kalium and
natrium, names suggested by the Germans Klaproth and Gilbert,
who thought that potassa and soda , labels for "impure
substances of commerce", were inappropriate sources for naming
elements.
This means that now you must memorize that the
abbreviation for potassium is K (kalium), and for sodium Na
(natrium).
Thanks to Hans von Zerssen of the Liebig Museum, Giessen.
Based on information from:
Oxford English Dictionary
S. Heilenz, Das Liebig-Museum in Giessen, Edition Giessen,
1996
Ringnes, J. Chem. Ed. (1989) 66, 731-738
Berzelius and Gilbert "Versuch einer lateinischen Nomenclature
für die Chemie, nach electrisch-chemischen Ansichten",
Annalen der Physik (1812) 42, p. 37
Berzelius "Essay on the Cause of chemical Proportions, and on some
Circumstances relating to them : together with a short and easy
Method of Expressing them" Annals of Philosophy (1814)
3, 362.
Thomson "Outline of Dr. Berzelius's Chemical Nomenclature",
Annals of Philosophy (1814) 4, 450.
copyright 2001, 2006
J.M.McBride



