|
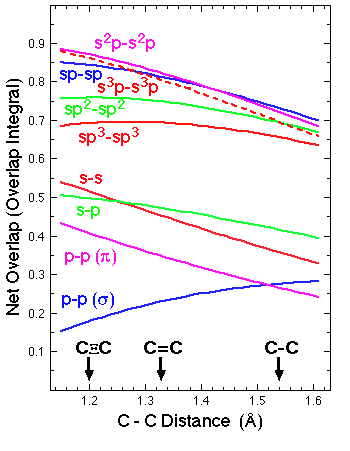
The plot shows the overlap between pairs of identical hybrid orbitals directed toward one another from adjacent carbon atoms at distances relevant to C-C bond formation. (It also shows overlap of a 2s orbital on one carbon with a 2p orbital on the other, and the side-by-side pi overlap of 2p orbitals.) Note that 2s-2p hybridized orbitals give roughly twice as much overlap as pure 2s or 2p orbitals. This is because hybrid orbitals concentrate to one side of the atom, where the two atomic orbitals reinforce Over most of this range sp (50%s ; 50%p) hybridization gives the best, or nearly the best overlap. (At the shortest distances hybrids with more s-character are even better.) Of course a carbon atom can form only two sp hybrids at a time, since each uses 50% of the 2s orbital. The advantage of sp3 hybridization is that is uses only 25% of the s orbital, and thus a single carbon atom can form four such hybrid orbitals. The resulting bonds are individually weaker because of poorer overlap, but there are more of them. There is no requirement that the s:p ratio in a carbon hybrid be precisely integral, and indeed it would be so only in symmetrical cases. (e.g. in symmetrical methane, CH4, the carbon would use exactly sp3, but for the bonds to H in unsymmetrical CH3F, it would use sp<3 for reasons we will discuss soon.) |
|
(Data from tabulation in Randic et al., Croatica Chem Acta, 1967, 39, 125)
|
|
|