Correlation Energy of Carbon in
Context
From Particles to Molecular Clusters
A case of
"Compared to
What?"
|
When we make the orbital approximation
for many-electron problems, even if we use a self-consistent
field treatment to try to take account of electron-electron
repulsion, we are doomed to overestimate the energy because
of neglecting the ability of electrons to stay away from one
another by correlating their motion. We dignify this error
by calling it "correlation energy."
How great an obstacle will correlation
energy present to our attempts to make sense out of organic
chemistry on the basis of orbital ideas? If the error is
tiny, we might well be justified in forgetting about
it.
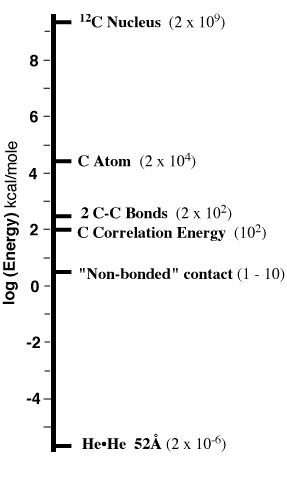
So let's think about the scale of
energies involved in matter and begin by taking everything
apart, right down to electrons, protons, and neutrons (leave
quarks for the physicists). The energies will span a range
of 1015 (from 2 mcal/mole to 2 billion
kcal/mole).
|
|
|
First we let protons and neutrons come
together to form a 12C
nucleus.
The mass of the carbon nucleus is slightly lighter than that
of its constituent six protons and six neutrons. Nearly 1%
of the original mass was converted to energy when the
nucleus formed.
This mass defect for carbon is
about 2 billion (2 x 109)
kcal/mole. Nuclear energy is quite
substantial!
|
12C atomic mass (C)
Electron mass (e)
Proton mass (p)
Neutron mass (n)
6 p + 6 n
12C - 6 e
Mass loss on forming C nucleus
Energy equivalent (e=mc2)
|
12 [by definition]
0.0005486
1.0072765
1.0086649
12.095649
11.996711
0.098938
2.13x109 kcal/mole
|
|
|
(Data
from NIST)
|
|
Now we add six electrons to the carbon
nucleus to make a carbon
atom.
This releases another 2 x 104
kcal/mole.
So the electronic energy of a carbon atom
is 105 smaller than its nuclear energy. A
small percentage change in nuclear energy would completely
swamp out any energy that is of chemical interest.
|
We know this by measuring the energy required for the
reverse process, removing the electrons one by one from the
carbon atom. Removing the first electron requires 11
electron volts of energy (multiply by 23.06 to get
kcal/mole). Removing the second electron requires another 24
eV; then 48, 64, 392, and finally 490 eV for the last 1s
electron. The total is 1030 eV or about 2 x 104
kcal/mole.
|
Ionization
Potentials
(eV)
11
24
48
64
392
490
Total 1030
|
|
(Data
Source)
|
Fortunately for us, once the nucleus is made, it
stays made, and carbon nuclei do not change their energy (and mass)
at all during addition of electrons to form the atom or during
chemical transformations. In comparing the energy of two chemical
structures, we do not have to worry about the contribution of nuclear
energy, because these humongous values precisely cancel out in any
comparison. We can leave nuclear energy to the nuclear physicists.
(Whew!)
Now let's do chemistry by bringing atoms together
to form a
molecule,
that is to form bonds. Carbon typically forms four bonds, each worth
about 100 kcal/mole. So a typical carbon atom participates in about
400 kcal/mole worth of bonds. If we assign half of the bond energy to
each of the two bonded atoms, we see that bonding generates about
200 kcal/mole per carbon atom. This is only about 1% of
the electronic energy that was released on forming the atom in the
first place. It is not surprising that x-ray diffraction shows that
atoms in molecules look mostly like pure atoms. Bonding is an almost
insignificant process compared to atom formation. This is also why it
is reasonable to use atomic orbitals as a basis for describing
molecules.
|
But for chemical transformations, bonding
is the name of the game. We absolutely must get these
energies right. Here's where correlation energy comes
in. If correlation energy (our mistake in estimating
electron energies) is of the order of 0.5% of the total
electron energy, that means this error is nearly as big as
bond energies!
|
Total Correlation Energy
Prof. Wiberg, who does heavy duty computations tells me
that in phenol, a molecule with 6 carbon atoms, 6 hydrogens,
and one oxygen, the correlation energy is 0.36% of the
energy difference between the molecule and the separated
atoms. In one sense this is a very modest mistake, but it
amounts to 687 kcal/mole - as much as 7 or 8 bonds!
|
How can we possibly tolerate such gross errors? We
might be lucky the same way we were with nuclear energy. If the
correlation energy (error) is identical in different arrangements of
the same atoms, we can neglect it in making the energy
comparisons
that are at the heart of chemistry.
|
Unfortunately, unlike nuclear energy,
correlation energy does change among different
bonding schemes. So to get really precise answers one needs
to do complicated calculations that go beyond SCF orbital
theory. But fortunately the changes in correlation energy
are modest enough that we can get very good qualitative
insight into how bonding works within the orbital
approximation. Changes in correlation energy during
most chemical transformations are usually less than 20
kcal/mole and often much less. So as long as we can
ignore errors amounting to 10 or 15% of a bond, we can use
orbitals.
|
Correlation Energy
(kcal/mole)
|
|
|
Phenol
H+ + Phenoxide-
|
687
701
|
Here are two examples of ionization of an organic
acid.
Notice that correlation stabilization is larger when
there is a lone pair in a single atomic orbital (as in the
phenoxide and benzoate anions) than when the same two
electrons form a bond in which most of the time they are on
different atoms.
(Thanks to K. B. Wiberg for data and
interpretation)
|
|
Change in C.E.
|
14.2
|
|
Benzoic Acid
H+ + Benzoate-
|
977
986
|
|
Change in C.E.
|
8.4
|
|
|
|
|
In Chem 125 we are using quantum mechanics
to get a reliable qualitative idea of what bonds are and why
they work the way they do. For this purpose we can put correlation
energy on the back burner and go ahead to use
orbitals.
(Trying to be more precise about correlation energy is
somewhat futile for practical purposes, because most organic
reactions take place in solution, and changes in the energy of
solvation between species under comparison are sometimes as large
as changes in correlation energy, or larger. Calculating solvation
energy accurately is at least as challenging a problem as
calculating correlation energy.)
Finally we come to a non-bonded
cluster
of molecules, a structure in which correlation energy plays a
dominant role.
Consider the world's weakest "bond", the He-He
"bond" in the helium dimer. This is
certainly not a normal bond, its strength is only 2 mcal/mole
(108 times less than a normal covalent bond), and the
average separation of the "bonded" helium atoms is 52Å,
34 times longer than 1.52Å, the length of a typical C-C bond.
This is not a "molecule," just a cluster of two atoms that would
quickly fall apart at temperatures above 0.001 Kelvin. No wonder
helium boils at 4.2 K.
The overlap of orbitals from different atoms is
energetically unfavorable when each of the overlapping orbitals
starts with two electrons, for a total of four. The overlap causes
two of the electrons (in the bonding "sum" orbital) to decrease in
energy. But the other two electrons (in the antibonding "difference"
orbital) increase in energy, a little more than offsetting the
decrease in energy of the bonding pair. This should make the atoms
repel one another. Of course at 52Å there is precious little
overlap within the He-He dimer.
So what holds the He-He cluster together?
Correlation in the motion of the electrons. Correlation energy is
sufficient to overcome the predominantly antibonding SCF energy of
He-He, which is of course tiny at a separation 52Å. The
electrons in one He atom tend to move in such a way as to be far from
the second atom when the electrons in the second atom are on the side
of their nucleus near the first atom. That is, if we denote electrons
by
:
and nuclei by
O,
situations A and B below are somewhat more common than C and
D
|
(A)
:O
:O
|
(B)
O:
O:
|
(C)
:O
O:
|
(D)
O:
:O
|
(Of course it is not so likely that the two electrons of
a single atom would happen to be on the same side of the nucleus.
They tend to avoid one another and stay on opposite sides, but for
the times when they are on the same side, A and B are more likely
than C and D.)
On average this generates a weak -+ -+ or +- +-
dipolar attraction between the two helium atoms that barely overcomes
the repulsion caused by the almost insignificant overlapping of
filled orbitals.
Analogous attraction occurs among all molecules,
and it is usually stronger, because other molecules have many more
electrons than the helium atom. But usually such "induced dipole"
interactions are worth only a few kcal/mole, 10-100 times weaker than
covalent bonding.
Although we spend most of our time in elementary
organic chemistry talking about normal bonding, these weak
attractions are very important in "supramolecular chemistry" and in
determining the physical properties of substances (things like
boiling, melting, flexibility, "recognition" of one biological
molecule by another, etc.).
copyright 2001-2003 J. M. McBride
