Planck Centennial and the Revolutions of
1900
It is amazing how profoundly the turn of 1900 to 1901
marked a transition from the 19th to the 20th Century in all sorts of
fields. Celebrating a centennial this December allows us to set in
perspective much of what we've been studying during the fall.
In International Politics: On
January 22, 1901, Queen Victoria died, ending the longest,
perhaps the dourest, reign in Britain's history, nearly two-thirds of
the 19th Century, and allowing her son Edward, whose love of fun
extended to popular
science lectures, to usher in the Edwardian era.
Trouble was already brewing with Victoria's grandson in
Berlin, Friedrich Wilhelm Viktor Albert, who was both King of
Prussia and Kaiser Wilhelm II of Germany. When Edward had
asked Victoria to give this troublesome nephew a "good snubbing",
she had replied, "Those sharp answers and remarks that only
irritate and do harm and in Sovereighs and Princes should be most
carefully guarded against. William's faults come from
impetuousness (as well as conceit); and calmness and firmness are
the most powerful weapons in such cases."
In Organic Chemistry: The
previous October Moses Gomberg, an Assistant
Professor at the University of Michigan, had published in Germany and
in the United States a paper entitled "An Instance of Trivalent
Carbon: Triphenylmethyl".
This paper marked the transition from the development of
structural organic chemistry over the last half of the 19th
Century, which depended on the tetravalent (and tetrahedral)
nature of carbon (our subject this
semester), to the development in the 20th Century of
understanding how reactions occur. During a reaction that changes
the set of four groups attached to a carbon, the atom must, at
least temporarily, be attached to either 3 or 5 groups, so these
unusual carbon valences play a key role in the reactive
intermediates of "mechanistic" chemistry (our
subject for next semester).
|
In Physics:
The most profound of these three events at the turn of the
century may have been a lecture to the German Physics
Society in Berlin on December 14,
1900, by Max Planck, professor of physics
at the University of Berlin, entitled
On the Theory of the Law of
Energy Distribution in the Normal
Spectrum.
This astounding paper changed the course of
physics and set the machinery in gear for finally
understanding chemistry at a fundamental level. From a
chemical perspective it is worth thinking about what was
behind Planck's suggestion that energy can be
quantized.
|

Max Planck (AIP)
|
A Difference between Matter and
Energy
In 1889 Lavoisier had included light and heat (Caloric) at the
head of his list
of elements. But as the 19th Century came and progressed it
became clear that there was a fundamental difference, besides the
possession of mass, between these "imponderable elements" and the
material ones: Matter was granular, energy was continuous.
The idea that matter is not smooth and infinitely divisible
was prominent during the whole 19th Century. It arose in 1801, when
John Dalton suggested the existence of atoms to explain the
failure of gases of different density to segregate. In organic
chemistry the atom concept underlay Berzelius's atomic weights
(Composition), Liebig
and Wöhler's radicals, Dumas's
types, and Couper and
Kekulé's valence
(Constitution, 1858). After Cannizzaro revived Avogadro's
hypothesis in 1860, everything started coming together in terms of
atomic and molecular reality, although young van't
Hoff's suggestion that it was meaningful to discuss the
arrangement of atoms in space (Configuration, 1874)
scandalized some of the older, more conservative chemists (like
Kolbe). Baeyer,
himself no stranger to the above concepts, refused to take seriously
young Sachse's
suggestion of unstrained boat and chair structures for cyclohexane
(Conformation, 1889). It would be 29 years (after the
structure of diamond had been determined by X-ray diffraction) before
Sachse's detailed picture was accepted, but by 1900 there was already
a long history of organic chemists believing in atoms.
The idea of matter being granular had also
been playing an important role in physics and physical chemistry for
the last half of the 19th Century, particularly in statistical
thermodynamics, where gas laws were interpreted on the basis of
the "kinetic theory" in which pressure resulted from the
collisions of atoms or molecules with each other and with the walls
of the container. In fact the average kinetic energy of the molecules
(Kav) was used to define an absolute scale
of temperature using the expression : T = 2/3
(Kav / k), where k came to be known as
Boltzmann's constant, which was chosen to make the new
temperature scale agree with the traditional Kelvin scale.
Mathematical mechanical analysis of these systems supported
"Equipartition of Energy", the
idea that at thermal equilibrium all atoms (or molecules) in a
mixture should have the same average kinetic energy (requiring the
lighter ones to be moving faster). Further mathematical analysis
allowed extending the equipartition concept to other types of motion,
including the rotations and vibrations of polyatomic molecules.
Statistical analysis of how energy should be distributed among
molecules or oscillators led Boltzmann to his famous law
showing that a "state" that is lower in energy than another one by
DH should be more likely to occur by a
factor of eDH/RT
(or at room temperature 103/4 DH,
where DH is in kcal/mole).
Why is it "statistical" to disfavor high energies?
Because if you have only so much energy to go around, it is
improbable to put large amounts of it into a few molecules of high
energy - better to divide the energy into many small portions that
can be distributed in a zillion different ways among many
molecules.
[This is closely related to why a floppy molecule,
like twist-boat cyclohexane, has a statistical "entropy" factor
that favors it over rigid chair cyclohexane, partially
mitigating the eDH/RT
advantage of the latter. There are simply many more ways of
arranging the twist-boat than of arranging the chair, in the
same way that there are more gauche- than anti-butanes. For
cyclohexane there are a lot more structures called twist-boat
than there are called chair.] (Click
for details)
In dealing with Avogadro's Number of particles, probability is
tantamount to certainty.
Statistics shows that margins of error are typically
proportional to the square root of the number of individuals in
a sample. When the New York Times polls 900 individuals,
the margin of error is 30 individuals (square root of 900), and
they quote a uncertainty of 3% (30/900).
If one were to poll Avogadro's Number of molecules (6 x
1024), the margin of error would be about 2.5 x
1012, which is large as a number, but represents
less that 10-10% of the population. So in chemistry,
if you know probabilities, you don't usually worry about random
statistical fluctuations. That's why repeatability is a
hallmark of good chemical experiments. Epidemiologists do not
expect such precise repeatabililty.
Using Boltzmann's relationship together with relative energies,
estimated by adding average bond energies (Ludimar Hermann,
1868), organic chemists can easily predict approximate equilibrium
constants for various transformations. Nowadays such energy
predictions are corrected for "steric energy" calculated by
molecular mechanics (remember Chem
3D).
By the year 1900 the atomic fine structure of molecules was
permanently entrenched in chemistry, though a few philosophically
rigorous physical chemists (like Ostwald) and physicists (like Mach)
were fighting a rear-guard action maintaining that all of this was
just a handy model that had not really been proven.
Bonding had something to do with energy, but ever since the French
chemists had blown dualism out of the water in the 1840s, there had
existed no fundamental physical theory of bonding.
Although it was clear that matter was discontinuous, kinetic
energy and heat and light seemed naturally to be continuous. You
should be able put an arbitrary amount of energy into a molecule, or
into a part of a molecule (like a bond vibration). Of course if you
put too much in, you might blow a molecule apart.
19th Century physicists became adept at dealing not only with
matter, and heat, and entropy, but also with electricity, and
magnetism, and light. Here they were also making rapid progress as
the turn of the century approached. It is relevant to our story that
they had developed a good, fundamental electromagnetic theory of
light allowing them confidently to predict how much light energy
an oscillating charge of a given frequency and given average energy
should emit in a given time period, and how readily it should absorb
light that was incident upon it. A key feature of this theory is that
the rate of light emission
is proportional to the amount of excess energy in the oscillator
and to the square of the oscillation
frequency, while its rate of light
absorption is proportional
to the amount of light at the relevant frequency but
independent of the value of the
frequency.
Rayleigh's Headache : The
"Ultraviolet Catastrophe"
Everything looked rosy, until June 1900, when the English
theoretician Lord Rayleigh decided to put these ideas together to
predict the amount of light at different frequencies in
equilibrium with matter at a given temperature. This was the
so-called "Black Body Radiation" or "Normal Spectrum".
This question had great practical as well as
theoretical importance, because in 1879 Edison had invented an
incandescent electric light bulb. [Actually
Edison's first patent (1868) had been for an electrical vote
recorder - Florida is really behind the times!] There
was a lot of interest in deveoping brighter, more efficient
illumination devices. In fact the Third Law of Thermodynamics was
discovered by Walter Nernst, a German physical chemistry
professor, in connection with his efforts to commercialize a new
kind of electric lamp. [Incidentally, these
efforts prospered and Nernst became so well-to-do that shortly
after Gibbs's death in 1904 he gave Yale a suitable bronze
memorial with a bust of Gibbs in high relief. You can see it in
the main entrance to Gibbs Laboratory.]
This appeared to be a straightforward problem. Equipartition of
Energy said that at equilibrium oscillators of different
frequency should all have the same average energy, while light theory
said that for a given energy oscillators of higher frequency
should radiate more light (proportional to the square of
the frequency).
If the higher frequency oscillators are radiating more light
energy, but are at equilibrium, they must be absorbing more light
energy as well. Since the probability of absorbing light at the
relevant frequency is independent of the frequency, the only way
to maintain the balance with emission is for there to be more light
at the higher frequencies, which seems to be no problem, since
more high-frequency light is being emitted.
Fine. Rayleigh had a clear prediction for makers of illumination
devices. Heating an object should make it emit more light at all
frequencies (all oscillators have more excess energy), and the amount
of light energy at a given frequency should be proportional to the
square of the frequency (as long as one maintains equilibrium by not
allowing the light to escape - think of the light inside a heated
hollow ball, where it cannot get away).
Whoa! This
says that at any temperature there is more radiation at higher
frequency (in proportion to frequency squared). So if there is any
radiation at all in the red or infrared (low frequencies - IR
frequency ~1013 / sec), there must be 10,000 times as much
in the ultraviolet (1015 / sec), and 1010 times
as much in x-rays (1018 / sec). Not to mention gamma rays.
If you look at any equilibrated object (even a cold one), the x-rays
and gamma rays it emits should fry your eyeballs.
This Ultraviolet Catastrophe is not only surprising, it is
also untrue.
For many years researchers in many places had measured the
spectrum of light emitted from heated bodies. The most recent and
most precise work came from physicists at the Berlin State
Physical-Technical Institute (PTR),
a laboratory devoted to solving industry-related problems, who
measured the overall light emission at different temperatures, the
frequency of maximum emission at different temperatures, and the
detailed shape of intensity vs. frequency curves at different
temperatures. This was fortunate for Planck, because he was professor
of theoretical physics in the University of Berlin, where he could
interact closely with the PTR experimentalists.
They had been finding curves that started in the infrared and at
first increased in intensity with something like the square of
frequency, but, as common sense suggests, they reached a maximum and
then died away at higher frequencies (see
below). As a sample was heated it went from having its
emission maximum in the infrared (invisible), to the red (red hot) to
the blue (white hot). (Click
to see the colors - but not their
brightness).
|
For example in 1898 Ferdinand
Kurlbaum at the PTR reported that the
overall radiation from a chamber
heated in steam was 0.0731 watts/cm2 greater than
that from the same chamber cooled in ice.
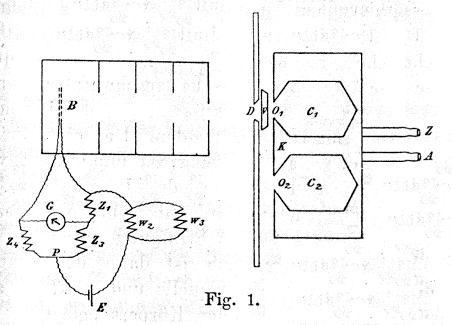
He made this measurement using the setup shown
in his Figure 1 on the right.
The box K was filled with either steam or ice
water (entering through Z, exiting through
A), so the chamber c1 came to
thermal equilibrium at either 100°C or 0°C.
When the little shutter V (held at room
temperature by circulating water and covered by velvet to
keep cold air from entering c1 through
o1) was pulled aside, the equilbrated
light inside c1 would shine through the
hole o1, and the aperture D,
onto the bolometer B, which would genenerate a
voltage that could be measured precisely with the
circuitry at the bottom.
This voltage measured the temperature of B, and
showed how much light energy it was absorbing from
c1 .
|
|
|
Others at the PTR noticed other cute things, such as
Wilhelm Wien's
Displacement Law. Not only does lm,
the wavelength of maximum emission, shift to shorter values
(higher frequency - toward the blue) at higher temperatures,
it does so in a very regular way, such that the product of
the absolute temperature and lm
is a constant.
Early in 1900 Otto Lummer and Ernst Pringsheim at the PTR
reported a value for the
Displacement Law
constant:
lmT
= 2940 mm
K
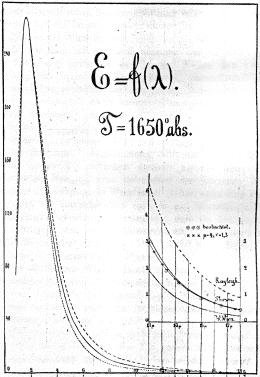
The figure to the right is Lummer and Pringsheim's
spectrum from a cavity heated to 1650 K. The vertical scale
is light energy; the horizontal scale is wavelength (from 1
to 18 mm in the infrared region
of the spectrum). Note that the maximum is at 2940/1650
= 1.78 mm
How could one understand these
careful data that differed so dramatically from the
predictions of "classical" physics?
|
(click for an
enlarged version)
|
Planck's Fabulous Insight
|
The first problem was to find an empirical
mathematical formula that would accurately reproduce the
shape of the curve of Emission vs. Wavelength for
different temperatures.
Wien came up with this "spectral equation" giving a
formula with two empirical constants, C1
and c2, that could be adjusted to give
more or less the right shape. Creating such an empirical
formula has obvious utility from the industrial, engineering
point of view, even if no one has any idea why it works.
|
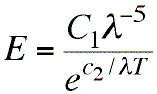
|
|
The purpose of the Lummer-Pringsheim paper of February
1900 was to show that the Wien formula wasn't quite right.
It gave the lowest curve in their figure above and deviated
substantially from their experimental points for long
wavelengths (see enlargement on the right of the
figure).
They found that the recent formula of Thiesen (also at
PTR) did a better job of fitting the long wavelength points
in their graph,
|
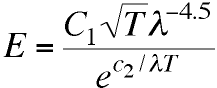
|
|
and that their own formula fitted the new points best of
all.
So far this was all just playing with formulas to
try to reproduce the experimental curves. Some formulas fit
one region of the spectrum well, some fit others, but none
fit the whole spectrum well.
|
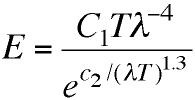
|
|
In a lecture on October 19, Planck weighed in with
his own improvement of Wien's equation - subtracting 1 from
its denominator.
Planck admired the presence of the
Boltzmann-like exponential term in this empirical
equation, and the integral exponents. He said that this
formula "seemed more likely to indicate the possibility
of a general significance than other previously proposed
formulas, apart from Wien's, which however was not
supported by the facts."
Overnight after Planck's lecture Heinrich Rubens, one of
the PTR researchers, checked Planck's formula against the
best available data, and found a good fit over the whole
frequency range.
Planck now tried to figure out what Nature was trying to
tell him by giving a curve with this shape. He was probably
not directly concerned with Rayleigh's headache, but he
wanted to develop a fundamental thermodynamic theory of
equilibrium that included light.
|
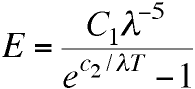
|
Within less than two months, he had figured it out.
The wildly incorrect "classical" prediction had been based on two
theories:
Statistical Thermodynamics (Equipartition of Energy), and
Electromagnetic Theory of Light (Emission at a given frequency
should be proportional to thermal energy and frequency squared;
Absorption should be proportional to amount of light at the
relevant frequency).
[So as not to become confused by
remembering this only partially in later life, note that the
total emission of light energy is proportional not to T but to
T4, because of how the spectrum shifts with
temperature. This is not relevant to the present
discussion.]
If the Theory of Light is correct, Equipartion of Energy must
be incorrect in predicting too much energy in high-frequency
oscillators (giving rise to the Ultraviolet Catastrophe).
Why might there be less energy in high-frequency oscillators at
equilibrium than equipartition predicts? Remember that statistical
thermodynamics was motivated by the need to avoid putting too much
energy in one place, because this would be statistically
unlikely.
Suppose that you could not put arbitrary amounts of
energy into an oscillator, that energy could be added to it only
in units of fixed size.
i.e. suppose that, at least as regards uptake
by oscillators, energy is granular (like matter).
Suppose that higher-frequency oscillators require energy to
be added in larger dollops, perhaps in amounts proportional to
their frequency (or maybe to the square of the frequency, or
to the square root of the frequency, or something).
If this were so, any energy to be added to high-frequency
oscillators would have to be added in large amounts, and
populating these oscillators would be statistically unlikely for
the exactly the same reason that large continuously variable
amounts of energy should not concentrate in individual oscillators
- just because it is not
a likely situation. [Of course at really high
temperatures more energy is available, and it becomes less
unlikely to localize a large dollop.]
So Planck tried assuming that the energy units e
that could be added to an oscillator were of size
hn,
where n (Greek nu) is the
oscillator's frequency and
h is his proposed
constant of proportionality between the frequency of an
oscillator and its energy unit. In a translation of Planck's words on
the evening of December 14, 1900:
"We treat E however - and this
is the most significant point in the whole calculation - as
composed of specific number of identical finite parts and make use
for that purpose of the constant of nature h = 6.55 x
10-27 [erg x sec]. This constant multiplied by
the common frequency n
of the oscillators [within a given family
of oscillators] gives the energy
element e
in ergs."
|
Using this assumption he redid the statistics to find the
most probable energy distribution among oscillators of
different frequency at a given temperature and to generate a
formula that is precisely equivalent to the empirical one he
had proposed on October 19, except for the translation from
wavelength to frequency and the replacement of the
adjustable constants C1 and
c2 by meaningful ones.
Notice the significance of the cube of frequency -
hn for the size of
the energy element, and n2
for the emission probability.
To generate numbers from his new theory Planck needed
only p, c
the speed of light (for converting from wavelength to
frequency), his new constant h (now called
Planck's constant), and k (Boltzmann's
constant).
|
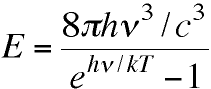
|
The value of p was well known,
as was c (Planck used 2.995 x 1010 cm/sec,
where we would use 2.998 x 1010). There were previous
estimates of k, but where would he get h?
By comparing his equation with experiment.
His equation showed that the maximum in the E vs.
l curve should in fact obey Wien's
formerly empirical Displacement Law with
lmT =
(c/4.965)*(h/k).
So he could use Lummer and Pringsheim's
lmT = 2940 mm
K to calculate h/k.
His equation also could predict
k4/h3 from
Kurlbaum's 0.0731 watts/cm2
difference in radiant heat between 100°C and 0°C.
So he didn't need the previous value of k. Instead
he found h/k
andk4/h3
and solved for h and k.The values of
these fundamental constants that he found in this way were correct
within 1% and 2.5%, respectively! (Lummer, Pringsheim, and
Kurlbaum at the PTR had done good work.)
He could check his value of k against previous
values for this constant. The agreement was not too bad
(though we now know that the previous values were off by 22%).
The ratio of R (the gas constant) to k
turns out to be Avogadro's number N. In this way Planck found
N = 6.175 x 1023, vs. 6.40 x 1023
by a previous method. We now know that Planck's value is better than
the previous one (in fact most of the error in Planck's N
comes from error in the literature value he used for R). He
could check against other things too, like the value of Faraday's
constant - the charge of a mole of monovalent ions.
So he was sure that he was right that, like matter,
energy could be quantized.
Sequel:
|
Energy quantization was a revolutionary idea, and not
surprisingly the whole world of physics did not immediately
stand and salute. Most physicists ignored his theory, and
Planck himself spent the next two decades trying
unsuccessfully to reach the same, to him obviously correct,
conclusion using the traditional ideas of physics.
In 1905, five years after Planck's proposal, Rayleigh
wrote "A critical comparison of the two processes
[his approach and Planck's]
would be of interest, but not having succeeded in following
Planck's reasoning, I am unable to undertake it."
But also in 1905 a very smart 26-year-old physicist
seized and extended Planck's idea. Whereas Planck had
only proposed that the amounts of energy an oscillator could
take up had to be multiples of hn,
young Einstein showed that light itself came in packets
of energy hn.
It was for this contribution, not for Relativity, that
Einstein would be awarded the Nobel Prize in 1921.
It would be another 21 years, after crucial intervening
contributions by other giants including Bohr, de Broglie,
Heisenberg, and many others, until Erwin Schrödinger
could explain WHY, and WHEN, the energy in matter should be
quantized. But the 20th Century was properly launched by
Planck on a path that would lead to understanding the source
of chemical bonding.
|
(AIP)
|
|
In 1918, the year Victoria's grandson Wilhelm II lost the
First World War, abdicated his throne, and fled to exile in
the Netherlands, Max
Karl Ernst Ludwig Planck (1858-1947), who was just 9
months older than Wilhelm, won the Nobel
Prize in Physics "in recognition of the services he
rendered to the advancement of Physics by his discovery of
energy quanta."
But Planck could not look back on this period with
unalloyed satisfaction, because, widowed since 1909, he had
lost his eldest son in the war in 1916, one of his twin
daughters in childbirth in 1917, and the other, also in
childbirth, in 1919.
In 1930 Planck became President of the Kaiser Wilhelm
Society for the Promotion of Science, but he suffered
greatly during the Nazi years, when he remained in Germany
because of his sense of duty but was openly opposed to the
govenment's policies, particularly those regarding the Jews.
In 1944 one of his two surviving sons, charged with
participating in an attempt to assasinate Hitler, was
executed by the Gestapo. Planck's home in Berlin was
destroyed by Allied bombing in 1945.
Planck died at the age of 89 in 1947, and the next year
the Kaiser Wilhelm Society for the Promotion of
Science was reestablished as the Max
Planck Society for the Promotion of Science, which
after more that 50 years survives and thrives as one of the
world's great scientific institutions, counting more than 80
Max Planck Institutes and 15 Nobel Laureates.
|
Literature Consulted (in addition to linked web sites):
F. Kurlbaum, Wied. Ann. d. Physik, 65. 746-760
(1898).
O. Lummer and E. Pringsheim, Verhandl. d. Deutsch. Physik.
Gesellsch. 2. 163-180 (1900).
M. Planck, Verhandl. der Deutschen Physikal. Gesellsch.
2. 202-204, 237-245 (1900).
M. Planck, Ann. d. Physik. 4. 553-563, 564-566
(1901).
Three Key Articles by Martin J. Klein:
Max Planck and the Beginning of the Quantum Theory, Archive for
History of Exact Sciences, 1. 459-479 (1962)
Planck, Entropy, and Quanta, 1901-1906, The Natural
Philosopher, 1. 83-108 (1963)
Thermodynamics and Quanta in Planck's Work, Physics Today,
19. 23-32 (1966).
Hans Kangro, Early History of Planck's Radiation Law,
Taylor & Francis, London (1976).
Encyclopedia Britannica (1967) articles on Victoria,
William II, Max Planck
Supplementary Material:
Planck
page of Prof. Robin Jordan (Florida Atlantic University)
If you're interested click to
(download a Power Point Presentation on Moses Gomberg) or to view
a (page on
Gomberg's life in Ann Arbor).
copyright
2000,2002 J.M.McBride

