Sachse's Strainless Rings (1890-93)
Conformational
Isomers
Baeyer suggested that both large and small
polymethylene rings should be strained, but Hermann Sachse, an
obscure 28-year-old assistant in Berlin, soon pointed out that large
rings need not be strained, because the carbons need not be coplanar.
Unfortunately he was not good at expressing this simple idea in terms
that organic chemists could easily understand, and he was far from
having Baeyer's clout.
In Berichte, 23,
1363-1370 (1890) he gave the following patterns, and directed the
reader to cut them out, fold them up, and paste a van't Hoff
tetrahedron on each of the darkened triangles. If one does this, the
first gives a clear model of the cyclohexane chair and the second,
the boat. (Note: Sachse called these isomers "symmetrical" and
"unsymmetrical", respectively. The silly but convenient names "chair"
and "boat" came later.)
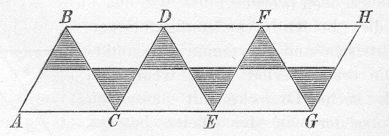
Sachse 1890 Chair Template
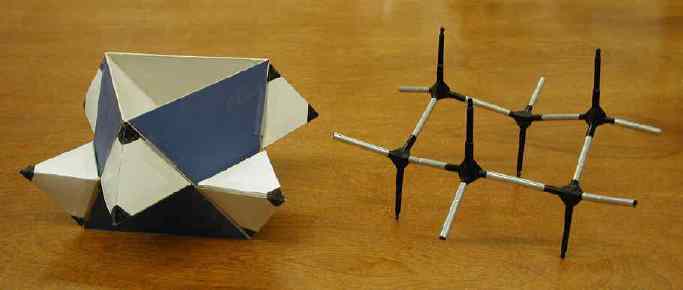
(Fold back along diagonal lines and tape A-B to G-H to make six faces
of an open ended octahedron. Realized in blue below.)
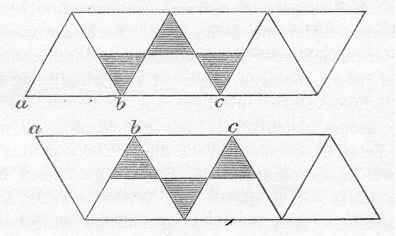
Sachse's Boat Template
(Fold each back along diagonals and tape edges to make six faces of
an open ended octahedron, then join the octahedra
through their open faces with a against a, b against b, and c against
c to make an hourglass. Realized in blue below.)
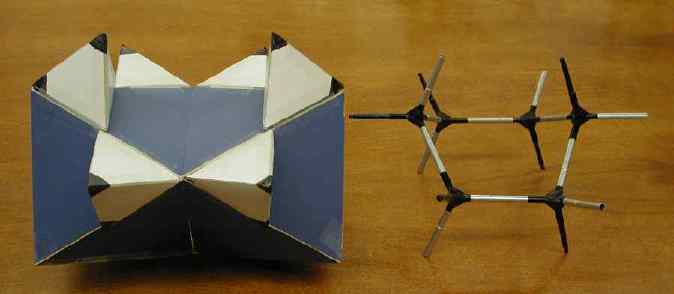
Sachse had it all worked out. He saw the axial
and equatorial positions for substituents. He saw how two chairs
could interconvert with a small barrier. He saw how certain
substituents might favor one of the chairs
Apparently Sachse didn't have enough clout to
make anyone follow his instructions. Thus Julius Wagner, who was
supposed to prepare a summary of Sachse's paper for the
Chemisches Central-Blatt (1890), wrote dismissively:
The author thinks he can clarify the easy
rearrangement of maleinoid hexahydromellitic acid into the
fumaroid without formation of the other possible forms by
hypotheses about the configuration [we would now say
conformation] of the hexamethylene ring.
It is not possible to write an abstract of this paper, especially
since the author's explanations are hardly understandable without
models.
Sachse was apparently
beneath Baeyer's notice, although
Baeyer "answered" his
criticism indirectly and perhaps
condescendingly in Liebig's
Annalen, 258, 145 (1890):
4) The Configuration of the
Hexamethylene Ring
A further proposal is that the atoms in
hexamethylene are arranged as in Kekulé's model, that is
that the arrangement of the atoms in space is the one with a
minimum distortion of the valence directions. Thus
the 6 carbon atoms must lie
in one plane and 6 hydrogen atoms lie in
each of two equidistant parallel planes. Further each of the 12
hydrogen atoms must have the same position relative to the other
17 atoms. The experimental test of the correctness of this
assumption is relatively easy, for example sufficient evidence is
that there is a single
isomer of hexahydrobenzoic acid
[i.e. cyclohexane carboxylic acid gives no
isomers]. Meanwhile, as long as our
knowledge in this field is so incomplete, we must be satisfied
that the above assumption is the most likely, and
no known fact contradicts
it.
Sachse knew he was right and
would not give up. In 1892 he supported
his point of view with a 41-page
trigonometric
paper [Zeitschrift für physicalische chemie, 10,
201-241 (1892)]. Page 216 is not atypical.
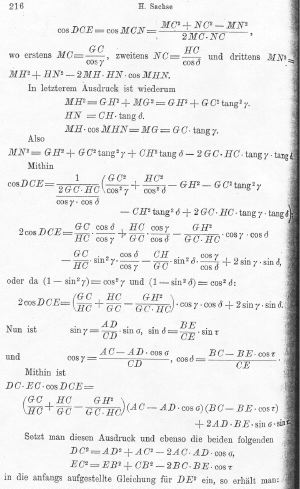
You can guess how far this got with an audience of
organic chemists!
He tried again in 1893 using
geometry
as well as trigonometry [Z. phys. chem. 11, 185-219
(1893)]. The following is representative of its 34
pages:
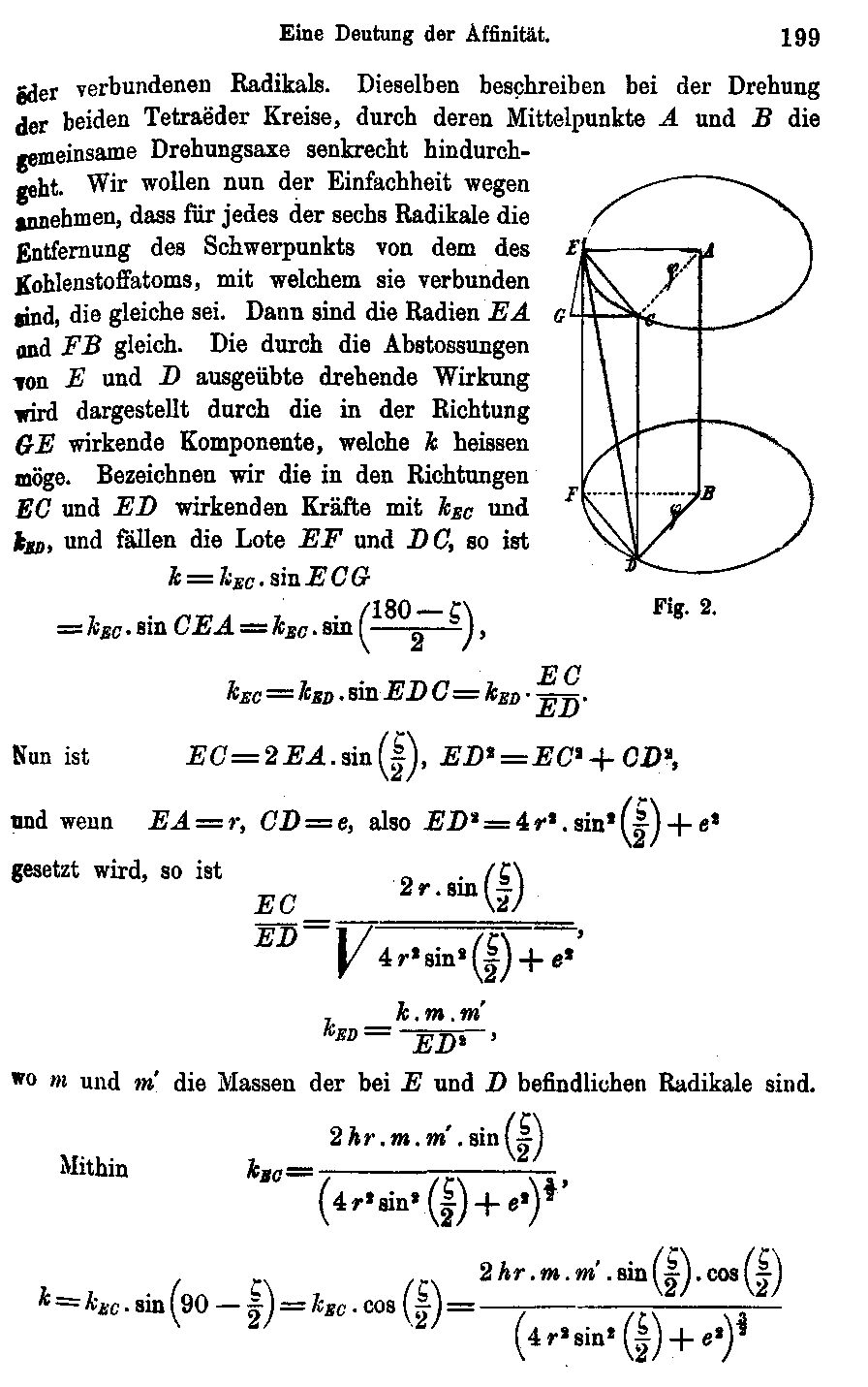
Again, good luck with an audience of organic
chemists!
Sachse died at the age of 31 in March 1893,
before this last paper was printed. His efforts had failed not because
he was wrong, but at least in part because
he did not express
himself clearly to the audience who
would have been interested in this perceptive contribution.
In his "Reminiscences" in 1905 Baeyer
wrote:
Sachse...
disagreed with my opinion that larger
rings are planar. He is certainly right from a mathematical
point of view; yet in reality, strangely enough, my theory
appears to be correct. The reason is not clear...
(translated
by R. Huisgen)
Only in 1918,
25 years
too late for Sachse, would his theory
be revived and proven correct by Ernst Mohr [J. Prakt.
Chem.[2] 98, 315 (1918)] on the
basis of the x-ray structure of diamond, which had been determined by
Bragg and Bragg in 1913 using X-ray diffraction.
Using the figures below,
drawn so clearly that
no one could misunderstand them, Mohr
showed cyclohexane within the structure of diamond.
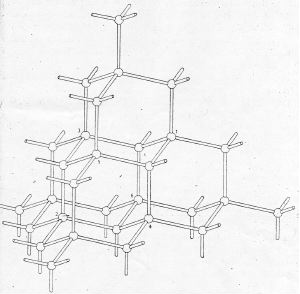
In Figure 1, Mohr showed Sachse's chair form
cyclohexne abstracted from the diamond lattice
(below) and by extension
imagined Sachse's boat form
(above):
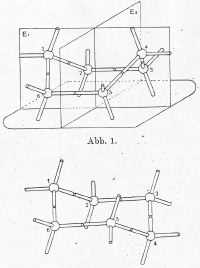
In diamond Mohr also saw the fused chair
cyclohexane structure of decalin:
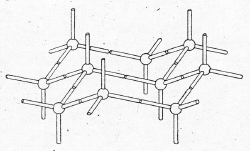
Surprisingly,
full recognition of
Sachse's premonitions would come only after half a
century, with a 1950 publication by
Britain's D.
H. R. Barton, whose conformational diagrams were much less accurate than Mohr's from 32 years earlier, or Sachse's of 1890. Since 1948
Conformational
Analysis has played a central role in
the theory of organic chemistry.
Lesson:
Know your audience, and
express yourself in terms they can understand.
For a fuller discussion of this
topic see Colin A. Russell "The Origins of Conformational Analysis"
in O. B. Ramsay, ed., "van't Hoff-Le Bel Centennial", ACS Symposium
Series 12, Am. Chem. Soc., Washington, D.C., 1975, pp.
159-178.
Thanks to Matt Weinschenk for
constructing the Sachse models.
copyright
2001 J.M.McBride