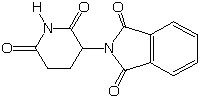

Thalidomide, first synthesized in 1953, was widely prescribed for morning sickness from 1957 to 1962, but only outside of the U.S. In 1961 the U.S. Food & Drug Administration was glad it had not given approval, because thalidomide became anathema when it was found to be seriously teratogenic (creating malformation in embryos, from the Greek for "monster") having caused serious birth defects in more than 10,000 babies. Now, a quarter of a century later, it appears that it may be a miracle drug for such diseases as AIDS, leprosy, lupus, and tuberculosis. Over the objection of groups focussed on the risk and seriousness of birth defects, in 1999 the F.D.A. approved its use with strict safeguards to protect women who are, or could become pregnant.
Since thalidomide has a stereogenic carbon atom, it exists as two enantiomers. Tests with mice in 1961 suggested that only one enantiomer was teratogenic while the other possessed the therapeutic activity. Unfortunately, subsequent test with rabbits showed that both enantiomers had both activities.
In one sense this is not surprising, because it is likely that the same mode of action is operative in both functions (in many cases it seems to be prevention of angiogenesis, the development of new blood vessels).In another sense the shared activity seems surprising, because one might expect the enantiomers to interact quite differently (diastereomerically) with the chiral, resolved molecules of nature.
The solution of this second riddle is that the enantiomers interconvert (the compound racemizes) under physiological conditions. Thus the drug is being sold as a racemate.
Thalidomide is very much in the news. A web search will yield hundreds of recent hits. Here are a few representative sites:
chiral chromatography Do look at this one which shows a chromatography trace for racemic thalidomide.
The solid stationary phase of the chromatography, a stable enzyme, is chiral and resolved. Therefore the enantiomers cling to it with different tenacity and spend different fractions of their time in the moving solvent, thus migrating at different rates and emerging at different times, i.e. enantiomers are resolved on this chiral column.The horizontal axis is time to emerge from the column (minutes, increasing to the right), the vertical axis is amount of material (measured by light absorption). The two large peaks are the enantiomers; the smaller peaks are impurities.
What would happen with an achiral column?
What would happen with an achiral column and a resolved chiral solvent?
Lewis A popular account of the role of chirality in drug development. Here's a relevant passage:
Food and Drug Administration (FDA) policy published in 1992 (Chirality, 4:338-340) strongly urges companies to evaluate racemates and enantiomers for new drugs. Even though in many cases a single-enantiomer drug is safer than a racemate, an effective racemate can still be marketed. "If a firm has data supporting the safety and efficacy of a racemate, we'd have to approve it," notes Thomas McGinnis, associate director of pharmaceutical affairs at FDA.
The FDA is interested in chirality for purposes of finding the safest, most effective pharmaceuticals. Lawyers are interested for purposes of extending or sidestepping patents by invoking a different form of matter (say replacing a racemate with a single enantiomer, such a change in technology and patents is called a chiral switch).
An analogous case which has both chemical/biological and patent implications involves different crystal forms of the same chemical substance. Someone like myself, who thinks that non-bonded interactions can be as significant as bonded ones, is very sensitive to the significance of differences in crystal form.
Celgene received FDA approval to market racemic thalidomide. (Note the warnings.) It is proving a goldmine for the company because of off-label cancer therapy applications. In 2004 a 50 mg capsule sold for $39 in the U.S., while a 100 mg capsule sold for $0.07 in Brasil.
Ritalin
Although Celgene's thalidomide is racemic, their "Focalin", licensed to Novartis, is a "refined form" of racemic ritalin. This is a chiral switch from ritalin, which is currently patented and marketed by others as a racemate and prescribed, many would save overprescribed, for attention deficit hyperactivity disorder (ADHD). Here is text from an earlier version of the Celgene web page:
Celgene's leading pharmaceutical in the Chiral area is d-methylphenidate hydrochloride, a chirally pure version of Ritalin®. Phase III clinical trials are currently being conducted to determine whether the risk-to-benefit ratio of d-MPH is superior to the non-chirally pure drug that is now commercially available.
Several years ago (1998) the corresponding page said
The Company has completed its Phase I/II clinical trial of the chirally pure formulation of d-methylphenidate (d-MPH). This study demonstrated that d-MPH, at one-half the usual doses of dl-MPH, is statistically superior to placebo in improving academic productivity and controlling the behavioral symptoms of children with ADHD. The Study also indicated that d-MPH, at one-half the usual dose of dl-MPH, may have a longer duration of efficacy than dl-MPH. Pivotal studies of d-MPH are planned to initiate in the second half of 1998.
The FDA website has label information for the Novartis Focalin (the d-isomer) as a PDF file at FDA Label Information. It is interesting to search this document for the character string "l-" to find references to the dl- or l- versions of the drug, and see what the tests showed about the relative efficacy of the single enantiomer versus the racemate (for example on p. 4). It would be interesting to compare the retail prices of the racemate and the single enantiomer.
Read the earlier Celgene claim above carefully to see what they are saying.
Do their test results, as described in the label, show that d-MPH is
more active than l-MPH?
If so, great. If not, why would they be seeking approval for the
single enantiomer?
If you ran the FDA what would you want to know?
Here is the structure showing the correct configuration of d-MPH:
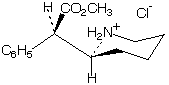
(What do you think of the conformation? We can wait a bit on this question.)
What does "d" mean in the name d-MPH?
You might enjoy giving this structure its CIP stereochemical descriptor (the carbon next to the CO2CH3 group is numbered 2, C6H5 is a phenyl ring) and naming the configuration of its stereoisomers (how many of them are there?).
|
|
|