Calculated Structure of Methyl Lithium
Tetramer
with a molecule of Dimethyl Ether
Because the lithium atom in CH3Li has three vacant orbitals, it is on the prowl to stabilize up to three high energy electron pairs in addition to those of the previously formed C-Li bonding electrons. This results in the following complex tetramer with an appended solvent molecule. This precise structure comes from a quantum-mechanical calculation (see footnote) but can also be observed experimentally.
Note that this structure can be
understood as a
tetrahedron of lithium atoms
interlaced with a
tetrahedron
of methyl carbons to form, overall, a distorted
cube.
The coloring in the following version of the picture shows how red
and maroon (CH3Li)2 dimer "squares" (analogous
to the B2H6 dimer of BH3) came
together along the blue bonds to form the "cube". Each blue (or red
or maroon) bond can be thought of as a lithium vacant orbital going
after a carbon HOMO to stabilize it. (In the square dimer the carbon
HOMO is already stabilized by red and maroon Li orbitals.)
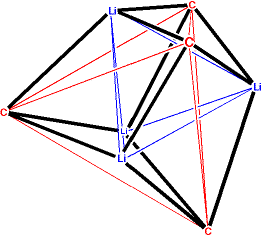
The oxygen of dimethyl ether forms a bond with one of the lithium atoms. Presumably if there were four ethers in the calculation, each lithium would be so decorated.
Although there seem to be 12 C-Li bonds within the distorted cube, there are only 4 electron pairs to accomplish this bonding (hence "electron-deficient" bonding). Each electron pair accounts for 3 of these bonds. Two of the four 4-branched version of the "Y" bond are shown by the blue and red lines added to the doctored figure below. Each of these can be thought of as the sharing of the "unshared pair" of a CH3 anion with three Li cations. Each lithium is involved in three such 4-branched bonds, while the fourth vacant valence-shell hybrid orbital of one lithium is used to stabilize an unshared pair of the oxygen in the ether at the right.