Ψ2
is actually Probability Density
When we said about Y
for a particular structure "the magnitude
of Ψ2
is proportional to the probability that the set of particles with
energy E will find itself in this structure", we chose the words
"proportional to" carefully. Ψ2
is actually probability density rather than
probability.
The difference is sort of pedantic, but
let's get in the habit of thinking clearly about graphs and
such.
This distinction may be clearer if we think
about mass density and mass, rather than probability
density and probability.
|
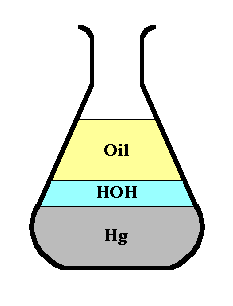
Suppose we had an Erlenmeyer flask
containing mercury, water, and oil, and that we were
interested in the mass within it.
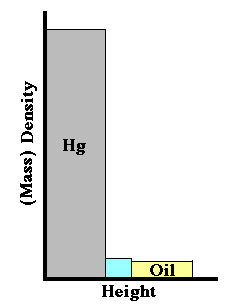
We could make use of the graph at the
right, but the proper label for the y-axis is (Mass)
Density, not Mass.
To go from Density to Mass one must
multiply by volume. (This is related to the
old conundrum, "Which weighs more, a pound of feathers or a
pound of steel?")
Although the density is high at any
point within the mercury layer, the amount of mass at
that point is zero, because a point has no volume.
You only get mass by multiplying density by volume, or
integrating the density function over a volume, if the
density varies with position.
[Note also that the plot
at the right, although correct, is misleading as to the
relative masses of the three liquids. It shows clearly
that there is more depth of oil than of water, and that
oil is less dense, but it doesn't take into account the
variation of the cross-sectional area of the flask with
height. The area of a bar in the plot shows the integral
of density over height, but not over volume, so it is not
mass (but rather
pressure).]
|
|
The same is true of probability. Suppose our
quantum mechanics problem had to do with the position of an electron.
For any given position, Y2
gives a certain probability density, but the probability of the
electron's being at that exact position is zero, because the point
has no volume. To find probability we must take account of volume
(either integrate the changing density over the volume in question,
or choose a small volume within which Y
does not change appreciably and
multiply).
We could properly compare probability densities
at two different points:
"The electron density in a 1s Hydrogen
atom is 10 times greater at the nucleus than 0.6 Å away from
it".
But to talk about absolute probability, we must
specify a volume:
"The Hydrogen electron spends 90% of
its time within a sphere of radius 1.4 Å", or
"The Hydrogen electron spends 5% of its time
in a region between 0.5 Å and 0.6 Å from the nucleus".
For a problem involving lots of particles, the
question of volume becomes pretty daunting, because the space has 3N
dimensions, where N is the number of particles, but still
Y2
is proportional to a probability density.
For example one could properly ask,
"What is the probability that a
triatomic molecule has each of its atoms within 0.05 Å of
its position in a particular arrangement?"
but giving a specific answer would require
integrating Ψ2
over 9 variables representing x,y,z motion of each of the three
atoms.
For obvious reasons, people very rarely ask
such questions for polyatomic molecules. We certainly won't in Chem
125! It is more practical to compare Ψ2
between two structures and say "The first arrangement is 10 times as
probable as the second", when you actually mean "The first has 10
times the probability density of the second."
copyright 2000
J.M.McBride