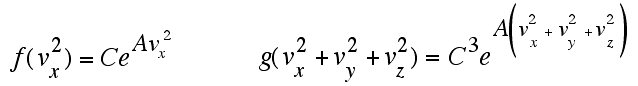The Importance of
Energy in Chemistry
James Clerk Maxwell (1859) /
Ludwig Boltzmann (1877)
In our attempt to understand the organic chemist's model of
molecules by finding out where it came from, we have been emphasizing
the importance of mass,
the
conservation of which allowed Lavoisier to launch modern chemistry on
a fruitful pathway and the analytical chemists of the 19th Century to
keep it going.
The other really crucial organizing principle is
energy,
which we are just
beginning to introduce into the molecular model in terms of average
bond energies and strain energies. Once energy is properly
incorporated into the the model we can use it to understand
structure, equilibrium, and reactivity, the most important concepts
of chemistry.
During all of our previous discussions (for example in quantum
mechanics) we have been making the implicit assumption that
molecules "want" to be in their lowest-energy state. For
example,
we've assumed that a reaction will take place in order to lower the
energy of the reagents (specifically to lower the energy of the
electrons in their orbitals, which is why bonds form by mixing HOMOs
with LUMOs or SOMOs with SOMOs).
This assumption is generally true (though sometimes
adjustments
are necessary to take "entropy" into account, as we'll soon see), but
why? What's so great about lowering energy? Why
don't
molecules strive for a particular color or shape instead of for low
energy? Why don't they, like industrious little entrepreneurs, strive
to have more energy rather than less?
The explanation is in the realm of thermodynamics.
"Thermodynamics" does not mean "the
dynamics of heat." The
term was coined in the mid-1850s to describe the efforts of
physicists to achieve a theoretical unification of the theory of heat
with the theory of kinetic energy and mechanics. A key paper entitled
"On the Kind of Motion We Call Heat"
was published in 1857 (the year before valence was
discovered). The idea was that heat involved the motion of
particulate matter (atoms or molecules) and that when you added heat,
the particles moved faster. Temperature was seen to be a measure of
average kinetic energy.
|
At first theoreticians tried finding the average
molecular velocity at a given temperature and assuming that all
molecules moved with this common velocity. This didn't get very far.
A great leap forward came when young James Clerk Maxwell tried
using a more realistic model in which the particles in a sample could
move at different velocities described by some probability
distribution. He wanted to find the
function f(vx)
that would describe the probability in a gas at
a certain temperature of finding particles with velocity vx
in the x direction. |

James Clerk Maxwell (1831-1879)
as a Cambridge undergraduate |
|
We
are dealing again with a probability density, the same concept we
encountered in describing electron distribution in space by the square
of an orbital. Now we are looking at probability density as a function
of velocity of an atom or molecule, rather than as a function of
position of an electron. Maxwell was a
very good mathematician. In 1854, when he graduated from Cambridge, he
had won second place in one university mathematics competition and
first in another. Now, five years later, he was able to determine the
probability distribution in such a beautiful way that it is worth
taking a moment to admire it, even in a course of organic chemistry.
|
In 1859 Maxwell wrote a revolutionary paper entitled "On
the
Motions and Collisions
of perfectly elastic
Spheres."
[This was just one year after organic chemistry was
revolutionized by the proposal of tetravalence and self-linking for
carbon by Alexander Scott Couper,
another Scot whose birth had been within 11 weeks and 35 miles of
Maxwell's]
|
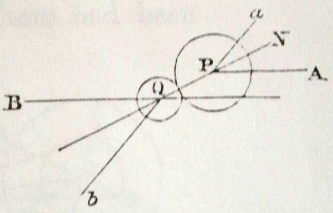
First Maxwell convinced himself that frequent random collision
among particles in a gas results in velocity distributions in
the x, y, and z
directions that are independent
of one another. That is, for a given particle the vx,
vy, and vz components of
its total velocity vT are independent of one
another. For
this purpose he drew diagrams of collision like the one at the right.
Don't worry about how he demonstrated the independence. He himself
worried about the argument and returned to prove the plausible
conjecture in a different way some years later.
Then, as shown below, Maxwell saw that in the process of
saying that these three components were independent, he had in fact
solved the problem of finding f(vx) ! This seems too easy, and in fact Maxwell revisited
the problem later to be absolutely sure he was right.
|  |
If the probabilites of vx,
vy, and
vz are independent, then the joint
probability of
having some particular set of component velocities for a particle of
a given mass is just the product of the individual probabilities.
That is, g(vT), the probability of a particular
three-dimenaional velocity
vector vT, must be the product of the component
probabilities:
g(vT)
= f(vx)
• f(vy)
•
f(vz)
Of course something that is a function of vx
is also a
function of vx2 (as
long as, like this probability, it doesn't depend on the sign of vx),
so we could equally
well have written (with different g and f functions):
g(vT2)
=
f(vx2) •
f(vy2)
•
f(vz2)
But [and this is absolutely key]
Pythagoras says that vT2
=
vx2 + vy2
+
vz2, so
g(vx2
+ vy2
+ vz2) =
f(vx2) •
f(vy2)
•
f(vz2)
That is, the function of a sum is equal to the
product of
functions of the components. There is only
one function that allows you to multiply by adding. It is the
exponential, where you add exponents in order to multiply. The
existince of the previous
relationship requires that f (and g) have the following form:
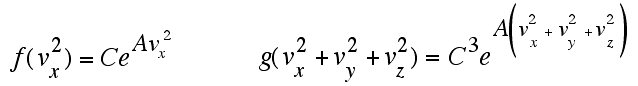
This says that, if the
component velocity distributions are
independent, the probability for having an x-component of velocity
between vx and vx+ dx
must be a constant (C)
times e raised to the power (A times the x-velocity
squared).
The x-velocity squared is of course proportional to the kinetic
energy, so the exponent involves kinetic energy for
motion
along x. Now we just need to find C and A.
The constant C just serves as a
normalizing constant (as in
orbitals) to make the total probability unity.
The constant A must be negative to give
finite
probabilities (otherwise the probability would increase without limit
with increasing velocity or kinetic energy). The magnitude of A (like
the Zeff when we looked at using effective
nuclear charges
in atomic orbitals) determines how rapidly the exponential decays.
The more negative A is, the less likely it is to have a high
vx. The size of A will determine the average
value of the
vx2. A is proportional to
1/T (where T is the
absolute temperature) and also to the mass of the particle, so that
the exponent is proportional to Ex/T,
where
Ex is the kinetic energy for motion along x. The
proportionality constant is 1/k. So we can rewrite in terms of the
kinetic energy rather than the velocity and plot the probability
distribution at a high temperature (red)
and a lower temperature (blue)
Note that no matter what the
temperature, the most likely
Ex (or vx) is zero!
For motion along x, or
y, or z, or any given direction the most likely single value of the
kinetic energy is zero.
In 1877 Boltzmann used a
completely
different
approach,
based on counting permutations, to
show that the same exponential distribution holds for any way in
which energy may be stored in a molecule (rotation about a particular
axis, vibration of a certain bond, promotion of an electron to a
higher-energy orbital, etc.).
k in the equation above is called the
Boltzmann constant.
If there are two different
ways of arranging
a set of atoms to give two different molecules, their relative
probability at equilibrium is given by their difference in energy,
ΔE:
K =
e-ΔE/kT
This is why molecules "want" to minimize their energy.
copyright 2003
J.M.McBride