Shooting off into
Space
Letter from Adolf Lieben (age 33) to Emmanuele
Patern˛ (age 22)
[Lieben's purpose in writing was to save his young friend and
former student from the embarrassment that would come from making the
rash suggestion that it might be meaningful to think about the
arrangement of atoms in space. Five years later the irascible
Hermann
Kolbe excoriated young J. H. van't Hoff for publishing a pamphlet
that dared to speculate about the arrangement of atoms (he even
attacked the man who translated it into German). The philosophical
content of Lieben's letter is almost identical to Kolbe's, but the
tone is quite different. Note that in the original manuscript the
word true is inserted as an
afterthought, since Lieben wanted to stress that he was not just
talking about the place you put a symbol in a formula.]
Reprinted by Patern˛ in Gazz. Chim. Ital., 43,
501 (1913) when he was about to give up its editorship.
Turin, 25 June 1869
My dear Emmanuele,
Let me comment on the isomerism which you and Cannizzaro have
pointed out between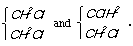
To imagine such an isomerism is certainly not absurd, even with no
difference among the 4 valences of carbon. However I don't believe I
err in supposing that the same reason which has kept me from adopting
it will also keep all the other chemists from doing so (in a silent
consensus).
Perhaps you have not thought that in assuming such isomerism you
have crossed the Rubicon which separates speculation, which is
considered permissible, about the way in which atoms combine from
speculation, which is less permissible, about the true positions of atoms in space.
In all previous speculation one has always been careful never to
consider the relative positions of the atoms in space (either
distance or surroundings). Such an idea does not even enter into
KekulÚ's speculations on isomerism in the aromatic series.
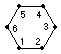 KekulÚ believes that
C6H4.1Cl.2Cl differs from
C6H4.1Cl.3Cl and from
C6H4.1Cl.4Cl because in
the first case the two CCl are joined to one another, in the second
separated by CH, in the third separated by CH.CH, and to say this one
does not need to hypothesize about the surroundings of the atoms.
Perhaps it could be that the two Cl atoms in the 1:2 arrangement are
in fact more distant from one another than in the 1:4! This does not
enter into KekulÚ's hypothesis.
KekulÚ believes that
C6H4.1Cl.2Cl differs from
C6H4.1Cl.3Cl and from
C6H4.1Cl.4Cl because in
the first case the two CCl are joined to one another, in the second
separated by CH, in the third separated by CH.CH, and to say this one
does not need to hypothesize about the surroundings of the atoms.
Perhaps it could be that the two Cl atoms in the 1:2 arrangement are
in fact more distant from one another than in the 1:4! This does not
enter into KekulÚ's hypothesis.
If you hypothesize on the relative positions of atoms in space you
would also make possible 2 CH2Cl2, even
granting the symmetry of the 4 valences of carbon and the existence
of a single CH3Cl, for example:
I repeat that I do not consider imagining such an isomerism to be
absurd, but since we have no means of knowing the topographical
position of the atoms (while we have many for knowing how they are
combined) I consider it a bit dangerous for science. Shooting off into space in search of atoms one risks
losing the ground under his feet! Best regards to Cannizzaro,
K÷rner, and Amato. I will soon write to Compisi.
Your good friend
Ad. Lieben
*/ Contrary to your hypothesis.
Click here to see an image of the manuscript
letter
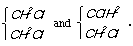
 KekulÚ believes that
C6H4.1Cl.2Cl differs from
C6H4.1Cl.3Cl and from
C6H4.1Cl.4Cl because in
the first case the two CCl are joined to one another, in the second
separated by CH, in the third separated by CH.CH, and to say this one
does not need to hypothesize about the surroundings of the atoms.
Perhaps it could be that the two Cl atoms in the 1:2 arrangement are
in fact more distant from one another than in the 1:4! This does not
enter into KekulÚ's hypothesis.
KekulÚ believes that
C6H4.1Cl.2Cl differs from
C6H4.1Cl.3Cl and from
C6H4.1Cl.4Cl because in
the first case the two CCl are joined to one another, in the second
separated by CH, in the third separated by CH.CH, and to say this one
does not need to hypothesize about the surroundings of the atoms.
Perhaps it could be that the two Cl atoms in the 1:2 arrangement are
in fact more distant from one another than in the 1:4! This does not
enter into KekulÚ's hypothesis.