|
Problem 1 Explain the formulas used in the
following passage from the Pepys/Newton webpage: 1 - (3/4)^6 = 0.822 , while the chance of at least double success with 12 tetrahedra is 1 - (3/4)^12 - 12 * (3/4)^11 * (1/4) = 0.842. |
|
|
Problem 2 Sketch a bar graph (analogous to the one to the right) showing the proportion of species M+4, M+2, and M (i.e. two Cl-37 atoms, one Cl-37 atom, and no Cl-37 atoms) for a substance whose molecules have 2 chlorine atoms. |
|
|
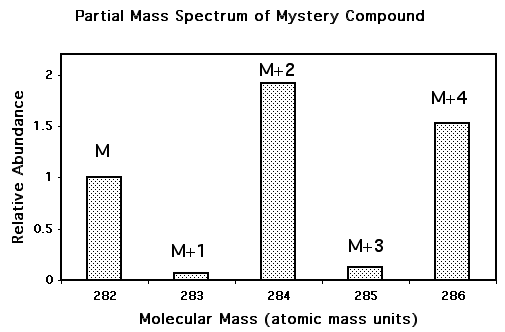
Problem 3 The bar graph to the right is a portion of the mass spectrum for a chemically pure "mystery compound" showing peaks at M, M+2, and M+4 due to the presence of chlorine (for purposes of this problem neglect the peaks at 283 and 285, which are due to small amounts of carbon isotopes). Note that the peaks are scaled to the mass 282 peak, which is taken as 1. Use formulas analogous to those in Problem 1 to determine how many chlorine atoms there are in a molecule of this compound. [It might help to work with a spreadsheet.] Knowing the number of chlorine atoms, guess what other atoms are present in the compound. [Note that M comes at 282 amu.] |
|
|
Problem 4 In an organic compound made from petroleum 1.07% of the carbon atoms have the mass 13 instead of 12. If none of the other atoms in the molecules have a significant probability of having a heavy isotope (which is not uncommon), it is possible to count the number of carbon atoms by observing what fraction of the molecules in the mass spectrogram have only C-12 atoms, and thus the nominal molecular weight, and what fraction are heavier because they have 1 (or 2) C-13 atoms. Suppose that the molecules of a substance have 30 carbons atoms. What proportion of the molecules should have 0, or 1, or 2 C-13 atoms if the source of the substance is petroleum? If the source of the 30-carbon organic molecule is a land plant with a "C4" metabolic process, the fraction of C-13 carbon atoms is 1.10%. What should be the proportion of molecules with 0, or 1, or 2 C-13 atoms in such a substance? How accurate would a mass spectrometer have to be to identify the source of such an organic compound (from petroleum or from a plant)? Special "isotope-ratio" mass spectrometers are used for this purpose (and even to distinguish between "C4" and "C3" classes of plants). When might it be useful to be able to determine whether an organic compound comes from petroleum or from land plants? |
|
Note