 |
 |
 |
One of the best ways to show you understand the conformation of hydrocarbons, and one of the best aids to clear thinking about this subject, is the ability to draw "realistic" perspective stick figures of cyclohexane. Drawing such figures does not require any more artistic skill than drawing a good Newman projection, but is does require understanding what you want to show, which is why it provides a good way of testing your mastery of conformational principles.
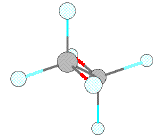
Perspective figures just use lines for bonds, and try to show the way a stick model with tetrahedral carbon atoms would look. For example the following three frames show ethane as a molecular model, a ball and stick model (from Chem 3D), and a perspective view. Note that anti bonds on adjacent carbons are parallel to one another in the staggered conformation (for example the bonds shown in red). Note also that bonds on a particular carbon which lie in the plane of the page (e.g. at 2 and 6 o'clock on the rear carbon) make a tetrahedral angle with one another, and other bonds make angles that look as though they would be tetrahedral in three dimensions.
 |
 |
 |
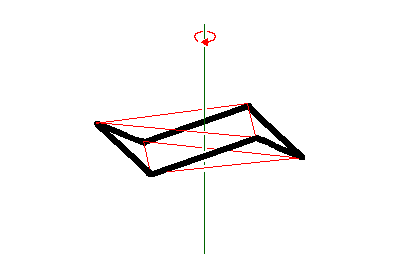
In drawing an idealized chair cyclohexane one first draws the ring of six carbons, usually as it would be seen from slightly above their mean plane. Note that the bonds that are parallel to the plane of the paper are slightly longer than the foreshortened ones that go in and out of the page. Note especially that opposite sides of the ring are parallel to one another.
 |
 |
 |
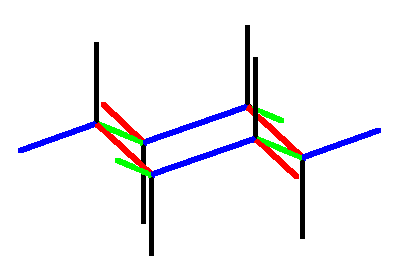 |
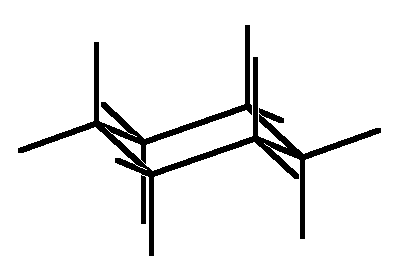
Then one adds the six black axial hydrogens, which are parallel to the vertical axis of 3-fold symmetry, shown as green in the frame on the top right, where the red triangles show which carbons are interconverted by rotation about this axis. Note that to make the carbons tetrahedral axial hydrogens must go up from the top carbons and down from the bottom carbons. Finally one adds the equatorial hydrogens, which, are anti to C-C bonds in the ring and thus parallel to them. Sets of parallel bonds are color coded in the bottom right frame.
It is easy to draw parallel lines. People who don't draw chair cyclohexane clearly fail not because they are not artistic, but rather because they don't understand the relationship among bonds in a low-energy staggered conformation. If you take the time to think clearly, you will draw clearly (with a little practice, see below).
 |
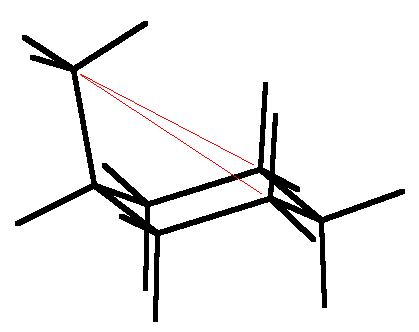 |
Actually cyclohexane drawn idealized is not quite correct. The C-C-C angles within CH2-CH2-CH2 hydrocarbon chains are actually 112°, not the tetrahedral 109.5°, so the cyclohexane ring is in truth flattened a bit [120° angles would give a perfectly flat hexagon]. Thus, as shown above left in the Chem 3D minimized structure, the axial hydrogens are actually splayed out a bit from being parallel to the 3-fold axis. Chemists usually ignore this fine point in drawing perspective views. With axial substituents, the gauche interactions (shown red) cause still more splaying, as shown for axial methylcyclohexane in the Chem 3D minimized structure above right. Again chemists tend to idealize their drawings of such structures. Note that the methyl group of methylcyclohexane is properly drawn in the staggered conformation
It takes a little practice and thought to draw cyclohexane correctly. This is clear from the mistakes made by the chemist who won the Nobel Prize for inventing conformational analysis of cyclohexane! (Click here to see if you can recognize the mistakes in his original drawing).
|
|
|